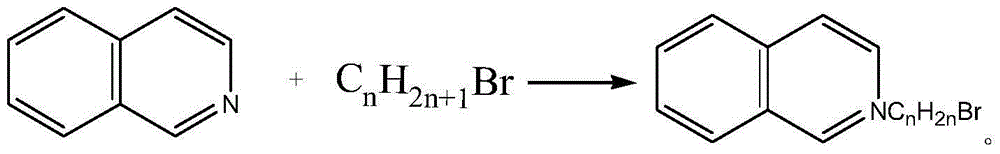Isoquinoline ionic liquid, synthesis and applications thereof
An ionic liquid, isoquinoline technology, applied in the application, botanical equipment and methods, chemicals for biological control, etc. High equipment requirements, easy separation and purification, and fewer by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Synthesis of Isoquinoline Ionic Liquids
[0025] 1)[C 4 Synthesis of iQuin]Br
[0026] Add 11g of isoquinoline and 10g of n-butane bromide quickly to a 50mL round-bottomed flask under anhydrous, oxygen-free, and nitrogen-protected conditions, stir and heat to reflux for several hours, and the product can be obtained at the end of the reaction. After cooling to room temperature, it was dissolved with solvent I, decolorized and impurity removed by chromatographic column, and then rotary evaporated to obtain wine red viscous liquid. After several times of recrystallization, a vacuum pump is used to remove the residual solvent with a low boiling point under an ultra-low pressure state to obtain a relatively pure brominated N-n-alkylisoquinoline ionic liquid (abbreviated as [C 4 iQuin] Br).
[0027] 2)[C 8 Synthesis of iQuin]Br
[0028] Quickly add 11g of isoquinoline and 17g of n-octane bromide into a 100mL round-bottomed flask under anhydrous, oxygen-free, and nitroge...
Embodiment 2
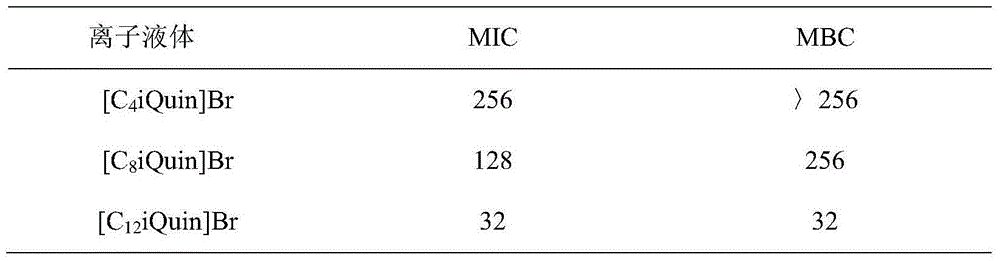
[0037] 1 The antibacterial activity of ionic liquid antibacterial agents was detected by the macro double dilution method.
[0038] Use a sterile inoculation loop to scrape off the bacteria on the slope to make a bacterial cell concentration of 5×10 6 CFU / mL bacterial suspension, culture and activate at 37°C for 12h. When in use, mix with an appropriate amount of liquid medium (the final concentration of bacteria is 10 5 CFU / mL). Dilute the sample to be tested to the required concentration by two-fold dilution. The negative control only contained bacteria; the blank control contained only culture medium. Each sample was cultured at 37° C. for 24 h in a shaker, and then plated for 24 h. Observe with naked eyes, record the number of colonies, and calculate the bacteriostatic rate, and the minimum concentration at which the bacteriostatic rate is above 80% is the MIC.
[0039] 2 Determination of antibacterial activity of ionic liquids on plant pathogenic bacteria by plate me...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com