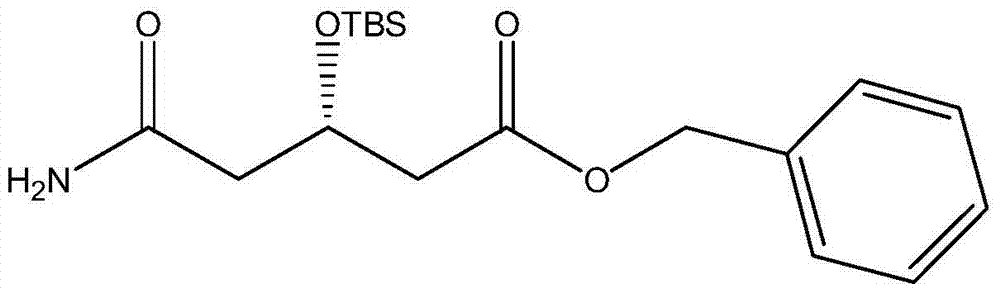
A kind of preparation method of (s)-tert-butyldimethylsiloxy-glutaric acid monobenzyl ester monoamide
A technology of tert-butyldimethylsiloxy glutaric acid monobenzyl monoamide and methanol, which is used in the blood lipid-lowering drug HMG inhibitor, tert-butyldimethylsiloxy-glutaric acid monoamide monomethyl In the field of ester preparation, it can solve problems such as difficult industrialization, chiral isomerization, and difficult separation, and achieve the effects of easy industrialization, less waste, and low process cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
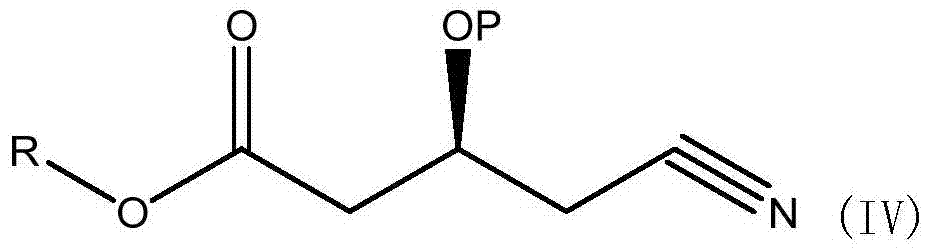
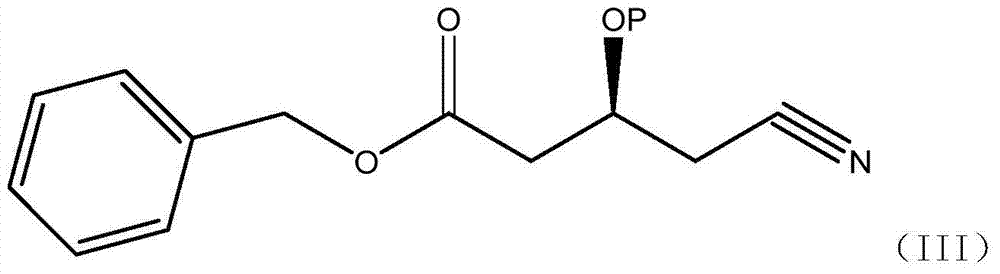
[0040] Dissolve 14.3g of (S)-3-hydroxy-4-cyanobutyric acid methyl ester (IV), 20g of benzyl alcohol in 250ml of toluene, NOVO-4353g, heat to 60°C, and reflux under reduced pressure for 12 hours, during which time remove and distill out A small amount of toluene, cooled, filtered, added 50ml of water to wash twice, concentrated organic phase, evaporated under reduced pressure to remove low waste point material to obtain (S)-3-hydroxy-4-cyanobutanoic acid benzyl ester, yield 96.5% (purity 92.4%).
example 2
[0042] Dissolve (S)-3-hydroxy-4-cyanobutanoic acid ethyl ester (IV) 15.7g, benzyl alcohol 20g, cyclohexane 250ml, NOVO-4353g, heat to 80°C, and reflux under reduced pressure for 16 hours, during which the Remove a small amount of cyclohexane that evaporated, cool, filter, add 50ml of water to wash twice, concentrate the organic phase, and evaporate the low-waste material under reduced pressure to obtain (S)-3-hydroxy-4-cyanobutyric acid benzyl ester , yield 98.2% (purity 90.6%).
example 3
[0044] Dissolve (S)-3-tert-butylsilyloxy-4-cyanobutanoic acid methyl ester (IV) 25.7g, benzyl alcohol 20g, toluene 250ml, heat NOVO-435 to 60°C, and reflux under reduced pressure for 12 hours. During this period, a small amount of cyclohexane evaporated was continuously removed, cooled, filtered, washed twice by adding 50ml of water, the organic phase was concentrated, and the substances with low waste points were evaporated under reduced pressure to obtain (S)-3-tert-butylsilyloxy-4 - Benzyl cyanobutyrate, yield 91.2% (purity 86.6%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com