A kind of omeprazole sodium 1,2-propylene glycol solvate and preparation method
A technology of omeprazole sodium and solvate, applied in the field of chemical engineering crystallization, can solve the problems of inconvenient aseptic operation in pharmaceutical production, need preparation of crystal seeds, etc., and achieves high crystal purity, short time consumption and high efficiency Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Add 3.00 g of omeprazole sodium amorphous powder to 10 mL of 1,2-propanediol and stir for 2 hours at 60°C. Then it was filtered, and the filter cake was dried at 60° C. and a vacuum of 0.05 MPa for 2 hours. Obtained 3.46 g of omeprazole sodium 1,2-propanediol solvate with a molar yield of 95.5%.
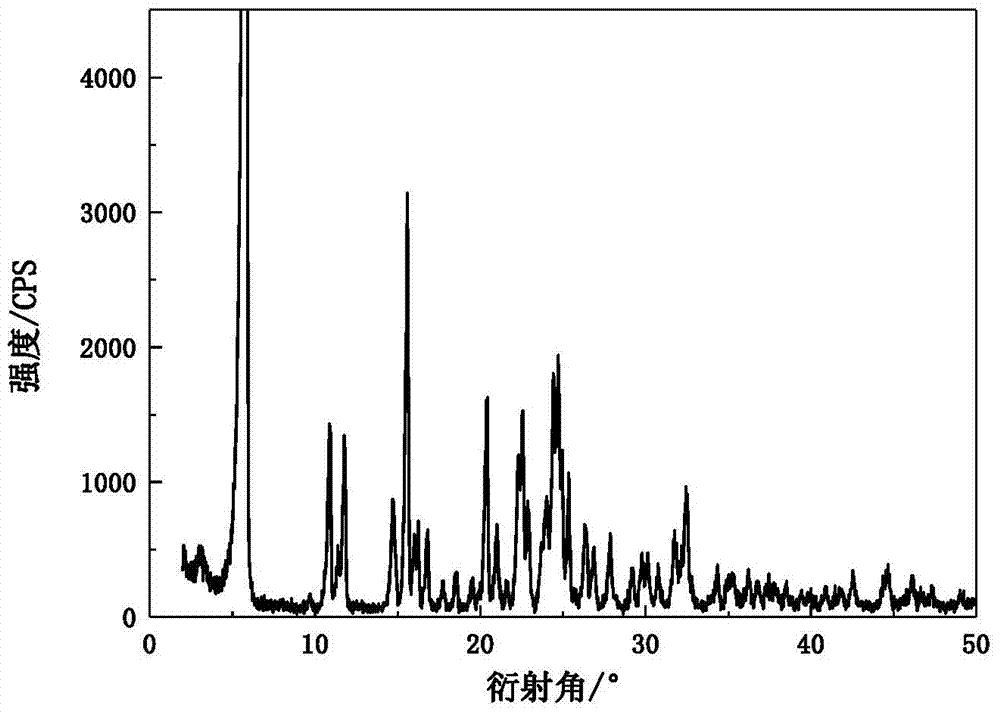
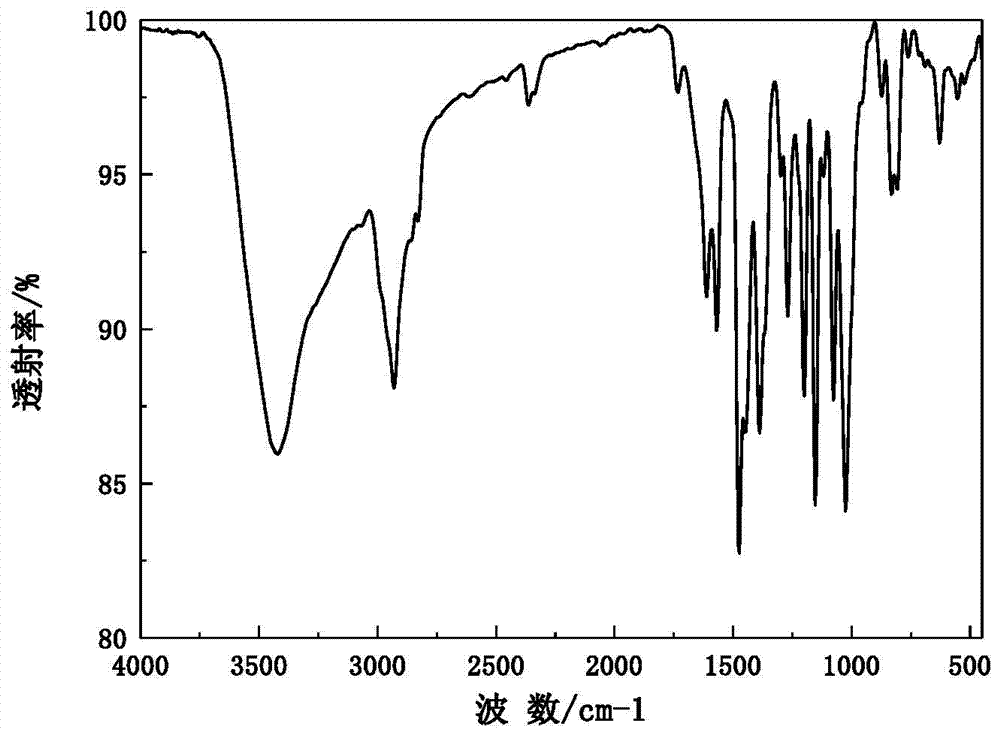
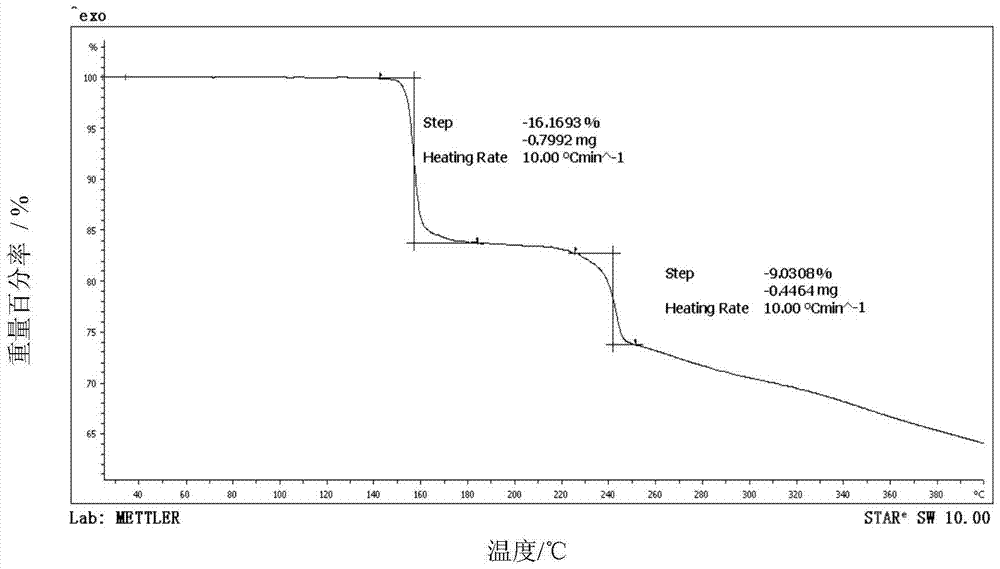
[0038] The X-ray powder diffraction pattern of the obtained omeprazole sodium 1,2-propanediol solvate and attached figure 1 Consistent, solid Fourier transform infrared spectroscopy and attached figure 2 In agreement, the DSC transition temperature is 150.1°C, the decomposition temperature is 220.6°C, and the main crystal grain size is 120μm, without coalescence.
Embodiment 2
[0040] Add 7.20g of omeprazole sodium monohydrate to 18mL of 1,2-propanediol, add 90mL of n-heptane at a time under stirring at 60℃, then continue to add 60mL of n-heptane at a rate of 1mL / min, and then continue stirring 5 hours. The crystal slurry is filtered, and the filter cake is dried at 20°C under normal pressure for 4 hours. Obtained 7.95 g of omeprazole sodium 1,2-propanediol solvate with a molar yield of 96.0%.
[0041] The X-ray powder diffraction pattern of the obtained omeprazole sodium 1,2-propylene glycol solvate at the diffraction angle 2θ was 5.90, 10.80, 11.72, 15.52, 20.32, 20.92, 22.58, 22.96, 23.92, 24.44, 24.64, 25.30, 26.32 , 27.86, 29.72, 31.74, 32.46 degrees have characteristic diffraction peaks, solid Fourier transform infrared spectroscopy in the wave number is 3421.3,2931.5,2362.2,1613.3,1567.6,1475.6,1389.6,1268.2,1201.3,1153.8,1077.4,1026.4,836.2 ,832.4,629.5cm -1 There is a characteristic absorption peak, the DSC transition temperature is 149.7℃, t...
Embodiment 3
[0043] 7.20g of omeprazole sodium amorphous powder was added to 14mL of 1,2-propanediol, and the temperature was lowered to 30°C at a rate of 0.5°C / min at 50°C with stirring. Add 15 mL of ethyl acetate at one time, and continue stirring for 3 hours. The crystal slurry is filtered, and the filter cake is dried for 4 hours at 40° C. and 0.08 MPa vacuum. Obtained 8.44 g of omeprazole sodium 1,2-propanediol solvate with a molar yield of 97.1%.
[0044] The X-ray powder diffraction pattern of the obtained omeprazole sodium 1,2-propylene glycol solvate at the diffraction angle 2θ is 5.82, 10.82, 11.74, 15.56, 20.30, 20.94, 22.56, 22.94, 23.94, 24.46, 24.68, 25.34, 26.34 , 27.86, 29.70, 31.76, 32.44 degrees have characteristic diffraction peaks, solid Fourier transform infrared spectroscopy in the wave number is 3422.8,2931.6,2364.1,1613.1,1568.6,1475.4,1387.9,1268.7,1201.6,1153.3,1075.8,1026.1,838.0 ,831.9,627.6cm -1 There is a characteristic absorption peak, the DSC transition tempe...
PUM
| Property | Measurement | Unit |
|---|---|---|
| decomposition temperature | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| decomposition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com