Docetaxel micelle drug load system and preparation method thereof
A technology of docetaxel and drug-carrying system, which is applied in the direction of pharmaceutical formulations, antineoplastic drugs, drug combinations, etc., can solve the problems of poor drug stability, no increase in tolerated dose, and low injection utilization, and achieve an increase effect The effect of strong power and good industrial application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Example 1 Polymer excipient mPEG 2000 -PLA 1800 -Synthesis of Phe (Boc)
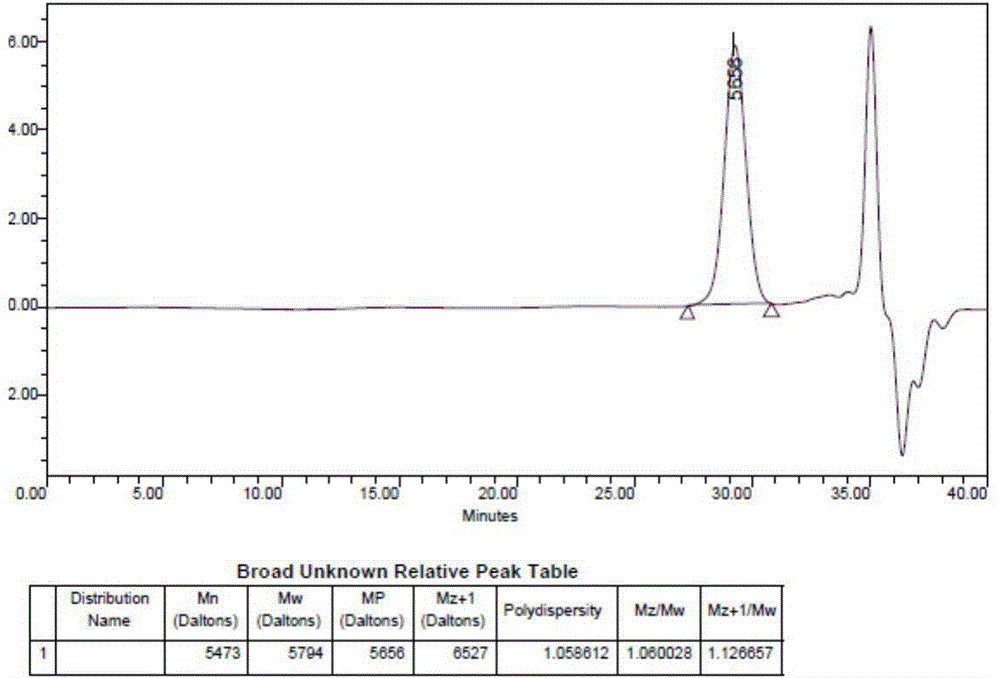
[0036] 20g (10mmol) of mPEG (number average molecular weight is 2000) was added to the polymerization flask, heated to 130°C and vacuum dehydrated for 3h before use. 1.28g (10mmol) of naphthalene dissolved in 20ml of tetrahydrofuran, add 390mg (10mmol) of potassium metal and stir at room temperature until the potassium metal is completely dissolved to obtain a dark green potassium naphthalene solution. Add the above solution to the dry mPEG, stir at room temperature for 5 minutes and then add 25g D,L Lactide / Tetrahydrofuran solution (1g / mL), stirred at room temperature under nitrogen protection for 30 minutes, vacuumed out the solvent, and then dissolved the reactants in ethanol. The resulting solution was cooled to -20°C and the precipitated polymer was filtered. MPEG 2000 -PLA 1800 Block copolymer.
[0037] 6.65g Boc-L-phenylalanine was dissolved in 50ml of anhydrous ethyl acetate, 4.2ml of triethyl...
Embodiment 2
[0040] Example 2 Preparation of docetaxel micelles
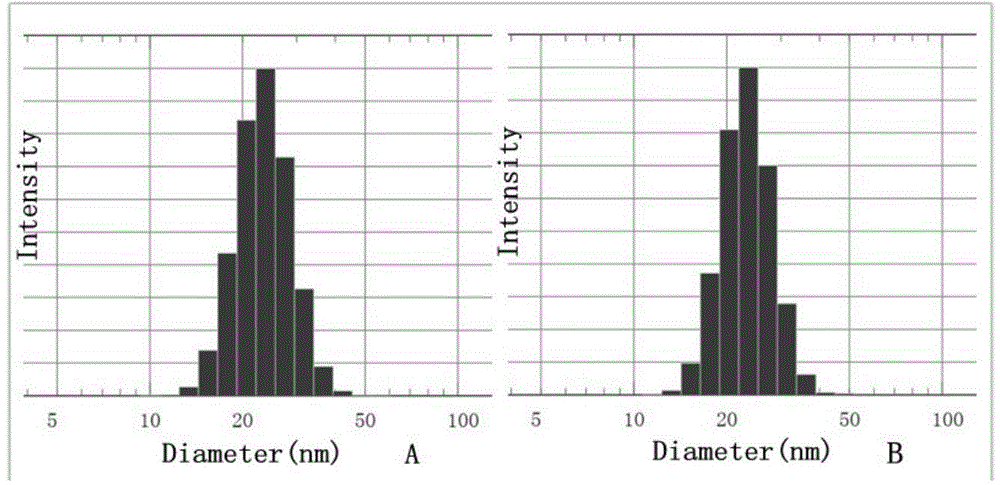
[0041] Take 100mg docetaxel and 1.9g mPEG 2000 -PLA 1800 -Phe(Boc) was dissolved in 25ml absolute ethanol, organic solvent was removed by rotary evaporation at 45℃, 25ml physiological saline was added to dissolve the drug film, 400mg mannitol was added and the solution was filtered through 0.22μm sterile film and then freeze-dried. Thaxa micellar freeze-dried powder. The LC-MS / MS analysis showed that the drug encapsulation efficiency was 100%, the drug loading was 5%, the particle size was 16nm, and the dispersion coefficient PDI was 0.02. Its resolubility is good, the particle size distribution changes little before and after resolubilization, the detailed results are attached figure 2 And image 3 .
[0042] Test Example 3 Pharmacokinetic test
[0043] A. Experimental animals:
[0044] Male SD rats, weighing 240±20g, were randomly divided into five groups, each with 6 rats, and spare.
[0045] B. Experimental preparation:
[0046...
Embodiment 4
[0057] Example 4 Pharmacodynamic test
[0058] 4.1 The inhibitory effect of docetaxel micelles for injection on xenograft tumors of prostate cancer PC-3A adriamycin-resistant cells in nude mice
[0059] Male BALB / c nude mice were inoculated subcutaneously on the ventral side of 5×10 6 PC-3A cells. About a week later, the average tumor size of the tumor-bearing mice reached 100mm 3 At the above, 30 tumor-bearing mice were randomly stratified and grouped according to the tumor volume, namely: vehicle group, docetaxel micelles for injection (10 mg / kg, from Example 2), docetaxel injection ( Taxotere, 10mg / kg), administered intravenously, once every 3 days for a total of 3 times. During the experiment, the animal tumor volume was measured every week (calculation formula ab 2 / 2, a and b are the length and width of the tumor respectively) and weight. The result is Figure 5 As shown, the micelle inhibition rate of docetaxel for equal-dose injection was significantly higher than that of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com