Medicine, namely, ranitidine hydrochloride composition capsule, for treating digestive system disease
A technology for ranitidine hydrochloride and digestive system diseases, applied in the field of ranitidine hydrochloride composition capsules for the treatment of digestive system diseases, can solve the problems of discoloration, easy deliquescence of ranitidine hydrochloride, poor stability, etc., and achieve Improve stability, solve the effect of easy deliquescence and simple components
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
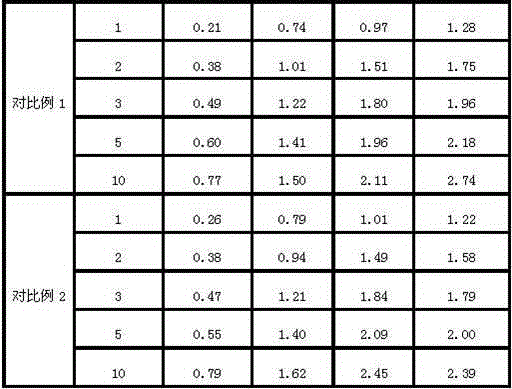
Examples
Embodiment 1
[0029] Example 1: Preparation of ranitidine hydrochloride crystals
[0030] (1) Add ranitidine hydrochloride solid into the mixed solvent of methanol, 2-methyltetrahydrofuran and acetonitrile, the volume of the mixed solvent is 10 times of the weight of ranitidine hydrochloride, the volume of methanol, 2-methyltetrahydrofuran and acetonitrile The volume ratio is 8:3.5:1.5;
[0031] (2) Control the temperature at 35-45°C, add a mixed solvent of acetone and ethyl acetate to the solution obtained in step (1), the volume of the mixed solvent is 9 times the weight of ranitidine hydrochloride, acetone and The volume ratio of ethyl acetate is 4:1.5;
[0032] (3) After adding a mixed solvent of acetone and ethyl acetate, cool down to -10-°C at a rate of 12°C / min, stand at -10-°C for 3 hours, precipitate crystals, filter, wash the filter cake with acetone, vacuum After drying, ranitidine hydrochloride compound is obtained.
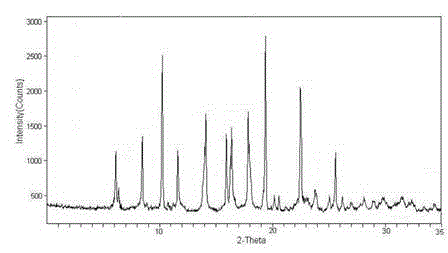
[0033] The X-ray powder diffraction figure that the prep...
Embodiment 2
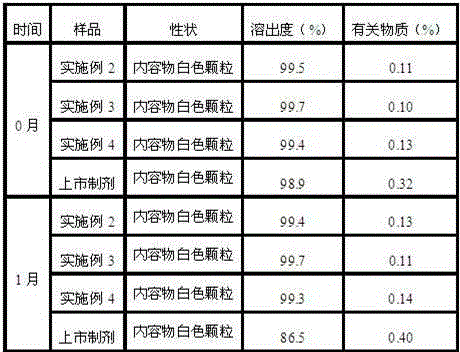
[0034] Example 2: Preparation of Ranitidine Hydrochloride Capsules:
[0035] Prescription: in parts by weight, 1.5 parts of the ranitidine hydrochloride compound prepared in Example 1, and 0.04 part of talcum powder.
[0036] 1) Raw material processing: sieve ranitidine hydrochloride with a vibrating sieving machine;
[0037] 2) Weighing: Weigh the raw and auxiliary materials according to the prescription;
[0038] 3) Mixing: Add the weighed raw and auxiliary materials into the mixer, set the operating frequency of the motor, and start the mixer to mix for 50 minutes;
[0039] 4) Filling by automatic capsule filling machine, the difference in filling volume must meet the national standard;
[0040] 5) Packaging.
Embodiment 3
[0041] Example 3: Preparation of Ranitidine Hydrochloride Capsules:
[0042] Prescription: in parts by weight, 1.5 parts of the ranitidine hydrochloride compound prepared in Example 1, and 0.05 part of talcum powder.
[0043] 1) Raw material processing: sieve ranitidine hydrochloride with a vibrating sieving machine;
[0044] 2) Weighing: Weigh the raw and auxiliary materials according to the prescription;
[0045] 3) Mixing: Add the weighed raw and auxiliary materials into the mixer, set the operating frequency of the motor, and start the mixer to mix for 50 minutes;
[0046] 4) Filling by automatic capsule filling machine, the difference in filling volume must meet the national standard;
[0047] 5) Packaging.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com