Crude drug bivalirudin purification process
A bivalirudin and process technology, which is applied in the field of purification process of bivalirudin raw materials, can solve the problems of low purity, low production capacity, unqualified and the like, and achieve the effect of reducing impurity content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0089] Embodiment 1, purification method of the present invention
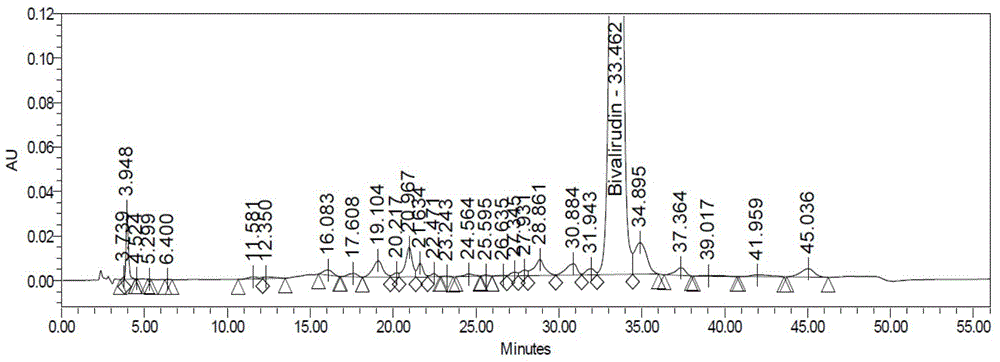
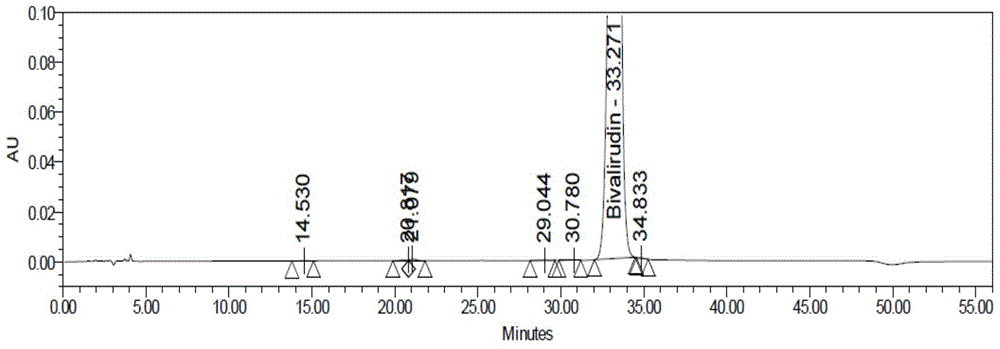
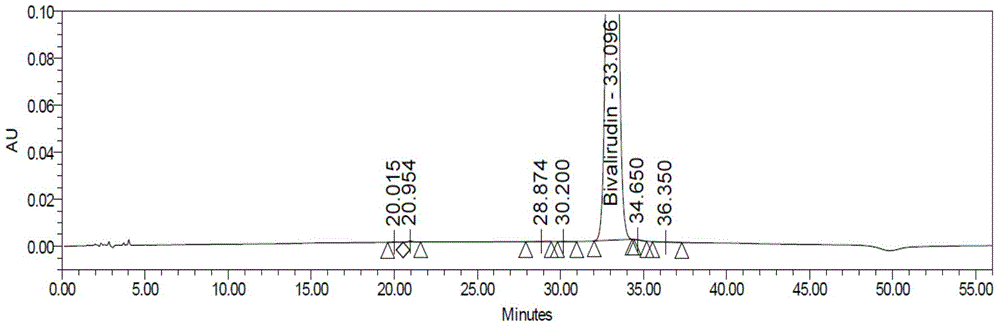
[0090] Step 1: Crude purity of bivalirudin crude product: bivalirudin crude product (provided by Hainan Zhonghe Pharmaceutical Co., Ltd., HPLC purity 87.47%, attached figure 1 ) was dissolved in purified water and prepared into a crude sample solution of corresponding concentration.
[0091] Instrument: Preparative High Performance Liquid Chromatography
[0092] Chromatographic column: C18, column diameter Φ15cm
[0093] Chromatographic column filler: 45μm, 100A
[0094] Mobile phase A: 0.1% heptafluorobutyric acid solution, mobile phase B: acetonitrile (chromatographically pure)
[0095] Wavelength: 230nm
[0096] Flow rate: 400ml / min
[0097] Elution gradient: mobile phase B was equilibrated with 5% for 15min, increased to 20% within 1min, maintained for 60min isocratic elution; increased to 50% within 1min, maintained at 50% for 5min, decreased to 5% within 1min, maintained until the elution ends.
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com