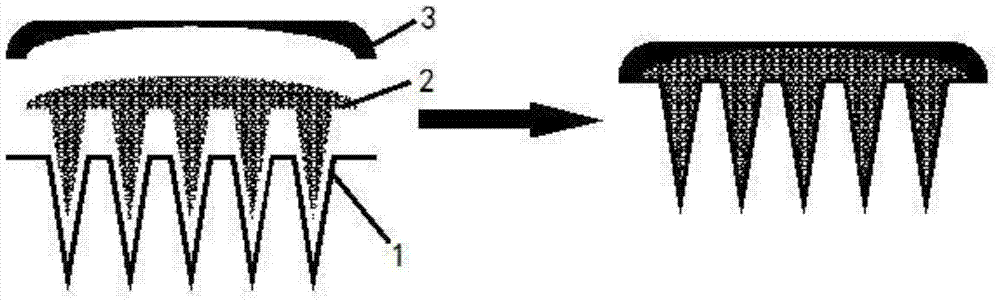
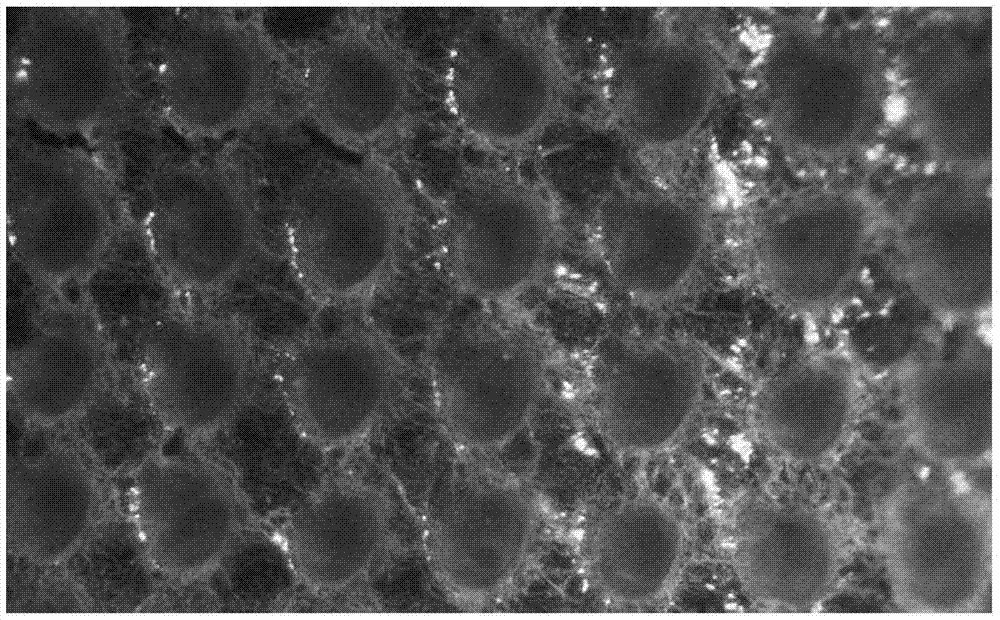
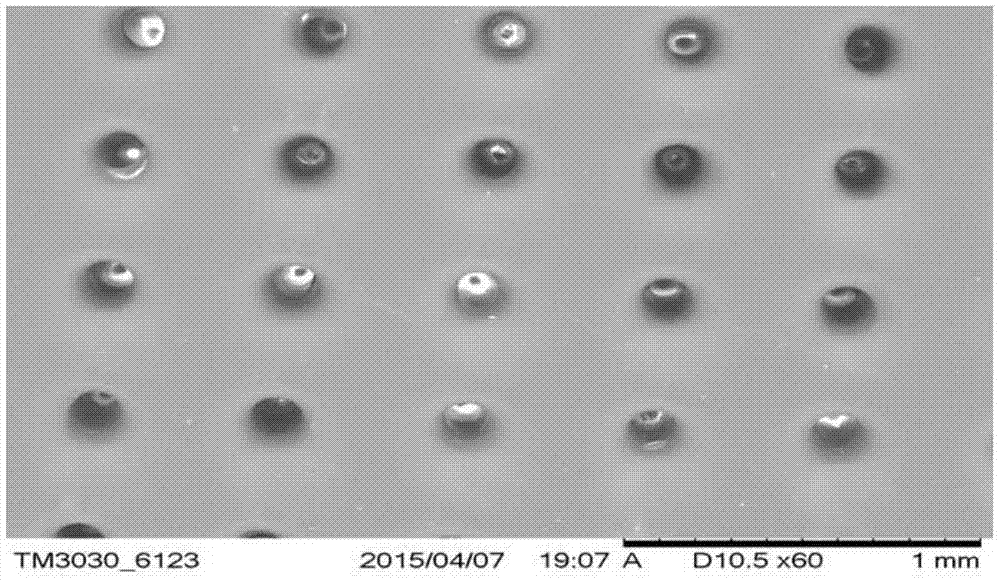
Swelling hollow silk fibroin microneedle drug delivery system and preparation method thereof
A drug delivery system and silk fibroin technology are applied to the swollen hollow silk fibroin microneedle drug delivery system and the field of preparation thereof, which can solve the problem of weak hollow microneedles with controlled release of drugs, low drug loading rate, difficult practical operation, etc. problems, to simplify the structure of the hollow microneedle transdermal drug delivery system, reduce skin sensitization and irritation, and facilitate sustained and controlled release.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment 1
[0034] (1) Preparation of silk fibroin solution: take 80g of silkworm silk and put it into 4000ml of 0.06% sodium carbonate solution, boil it three times at 98-100°C, and use deionized water for three times, each time for 30min, remove The sericin in the raw silk is washed, loosened and then dried in an oven at 60°C to obtain pure silk fiber. Dissolve the pure silk fiber obtained by drying in 9.3mol / L lithium bromide solution at 60°C, the bath ratio is 3:20, and the dissolution time is about 1h. After cooling, take it out and put it into a dialysis bag and place it in deionized water Dialyze for 3 days, filter with absorbent cotton to obtain a pure silk fibroin solution, concentrate it at room temperature, and put it in a 4°C refrigerator for later use.
[0035] (2) Modification of silk fibroin solution: the small molecule swelling agent glucosamine hydrochloride is diluted to 0.2g / mL with pure water, and the pure silk fibroin solution in (1) is in mass ratio (M swelling agent...
specific Embodiment 2
[0040] (1) Preparation of silk fibroin solution: take 80g of silkworm silk and put it into 4000ml of 0.06% sodium carbonate solution, boil it three times at 98-100°C, and use deionized water for three times, each time for 30min, remove The sericin in the raw silk is washed, loosened and then dried in an oven at 60°C to obtain pure silk fiber. Dissolve the pure silk fiber obtained by drying in 9.3mol / L lithium bromide solution at 60°C, the bath ratio is 3:20, and the dissolution time is about 1h. After cooling, take it out and put it into a dialysis bag and place it in deionized water Dialyze for 3 days, filter with absorbent cotton to obtain a pure silk fibroin solution, concentrate it at room temperature, and put it in a 4°C refrigerator for later use.
[0041] (2) Modification of silk fibroin solution: the small molecule swelling agent dimethylformamide was diluted to 0.1g / mL with pure water, and the pure silk fibroin solution in (1) was mass ratio (M swelling agent / M silk ...
specific Embodiment 3
[0046] (1) Preparation of silk fibroin solution: take 80g of silkworm silk and put it into 4000ml of 0.06% sodium carbonate solution, boil it three times at 98-100°C, and use deionized water for three times, each time for 30min, remove The sericin in the raw silk is washed, loosened and then dried in an oven at 60°C to obtain pure silk fiber. Dissolve the pure silk fiber obtained by drying in 9.3mol / L lithium bromide solution at 60°C, the bath ratio is 3:20, and the dissolution time is about 1h. After cooling, take it out and put it into a dialysis bag and place it in deionized water Dialyze for 3 days, filter with absorbent cotton to obtain a pure silk fibroin solution, concentrate it at room temperature, and put it in a 4°C refrigerator for later use.
[0047] (2) Modification of silk fibroin solution: the small molecule swelling agent biuret is diluted to 0.07g / mL with pure water, and the pure silk fibroin solution in (1) is mass ratio (M swelling agent / M silk fibroin ) = ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com