Glucose hydrogenolysis catalysis Cu/MgO catalyst and preparation method thereof
A glucose and catalyst technology, which is used in the field of Cu/MgO catalysts and preparations for catalyzing the hydrogenolysis of glucose to produce high value-added chemicals. It can solve the problems of high local concentration of active components, low product single selectivity, and small specific surface area of catalysts. problems, achieve broad industrial application prospects, solve the effects of poor selectivity and excellent catalytic performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~3
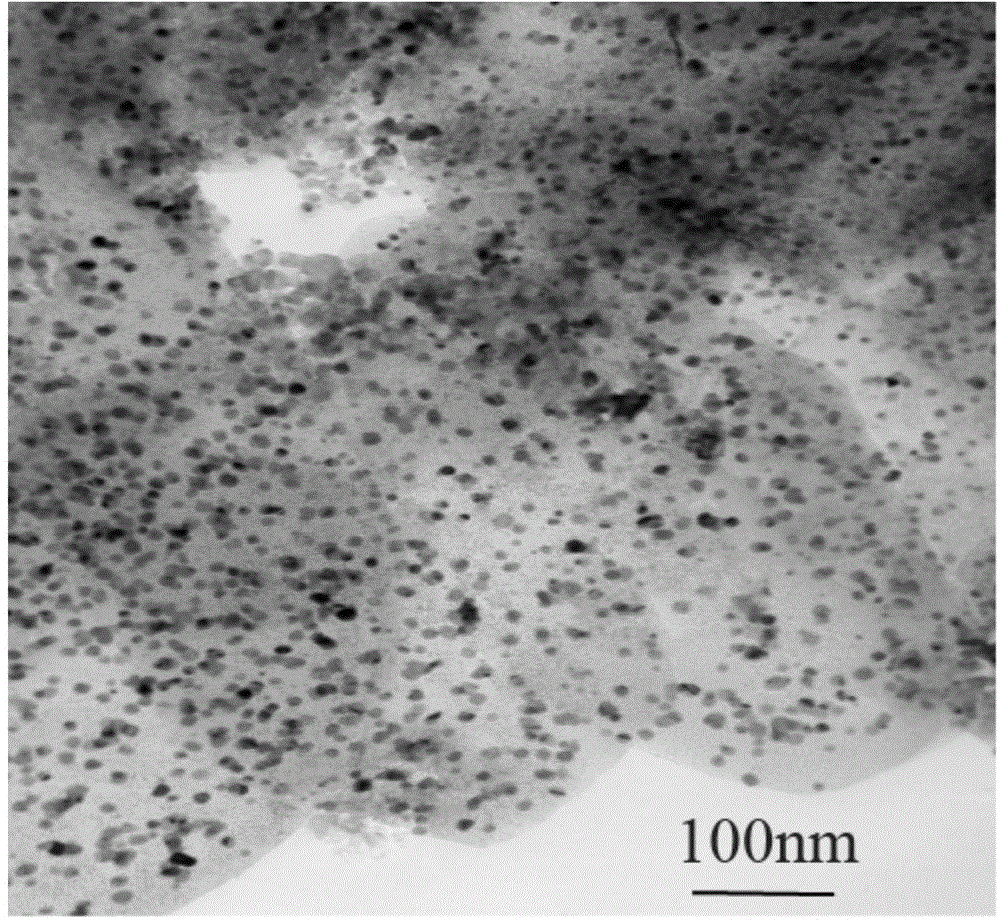
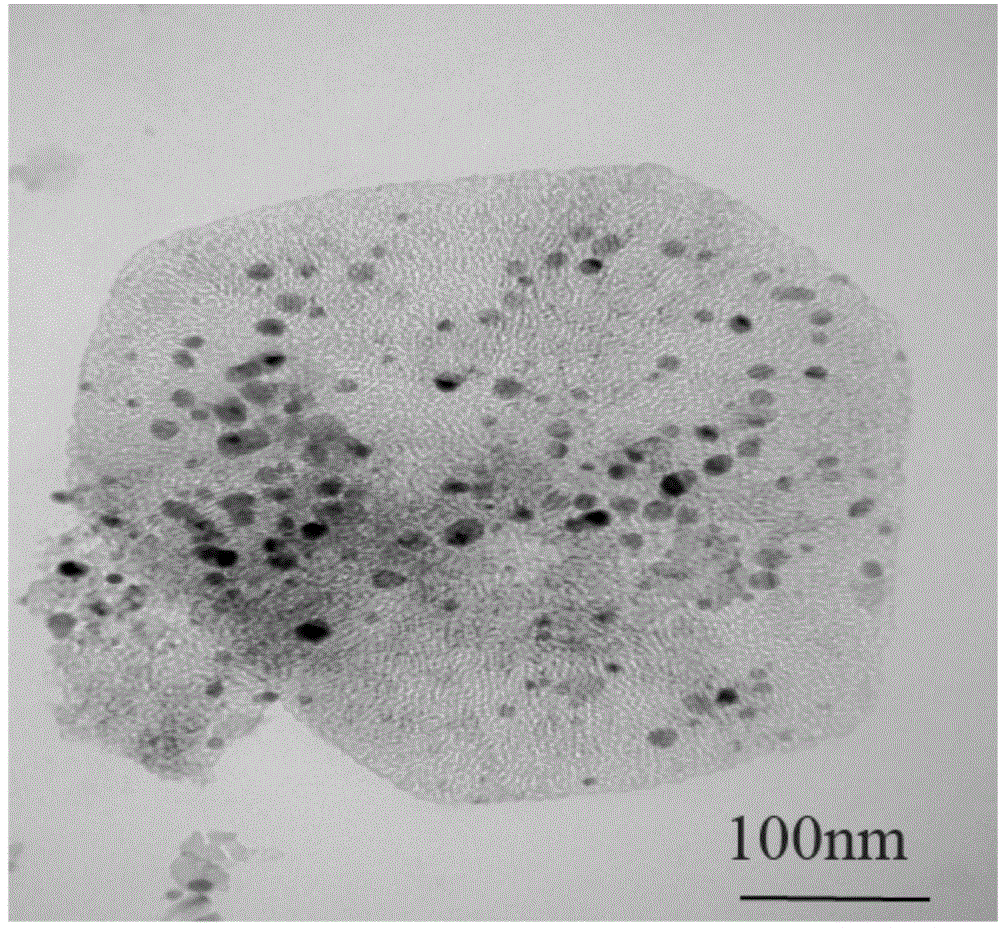
[0017] Add magnesium acetate tetrahydrate to a mixed solution of 60ML deionized water and absolute ethanol (volume ratio: 2:1, 1:1, 1:2 respectively) and stir the reaction, then add the solution to the stainless steel high pressure placed in an oven at 180°C to continue the reaction, and then the reaction solution was centrifuged, washed with corresponding proportions of deionized water and absolute ethanol, and finally dried in an oven to obtain Mg(OH) 2 , and placed in a tubular resistance furnace, calcined at 500°C in an atmosphere of argon and oxygen to obtain the MgO carrier. Considering the morphology and yield of MgO, deionized water and absolute ethanol with a volume ratio of 1:2 were selected for the reaction. The scanning electron microscope photographs of the obtained samples are shown in figure 1 .
[0018]
Embodiment 4
[0020] Will Cu 2 O was added to a three-neck round bottom flask, 1,5-cyclooctadiene and THF were added under an argon atmosphere to stir the reaction, and then 1,1,1,5,5 , 5-hexafluoro-2,4-pentanedione and THF continue to stir and react, and filter unreacted Cu after the reaction 2 O, the resulting filtrate is distilled under reduced pressure to remove the solvent, and then the solid powder is purified by sublimation to obtain the corresponding metal organic precursor hexafluoroacetylacetone-cyclooctadiene copper (I), which is obtained by thermogravimetric analysis, The loading temperature of the monovalent copper precursor was selected to be 100°C.
Embodiment 5
[0022] Basically the same as in Example 4, except that 1,5-cyclooctadiene is not added, the corresponding metal organic precursor copper hexafluoroacetylacetonate can be obtained. According to thermogravimetric analysis, the loading temperature of the divalent copper precursor was selected as 60°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com