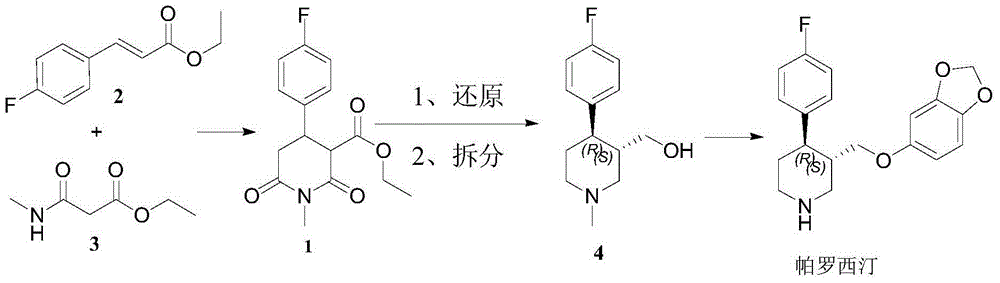
Method for synthesizing paroxetine chiral intermediate
A chiral intermediate and chiral technology, applied in the direction of organic chemistry, can solve the problems of high price of chiral inducing reagents, no application value, high price of chiral alcohol, etc., and achieve high practical value and social and economic benefits, reaction The effect of mild conditions and high reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
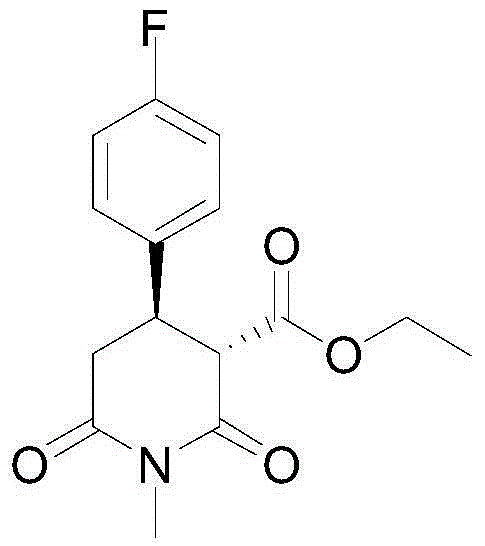
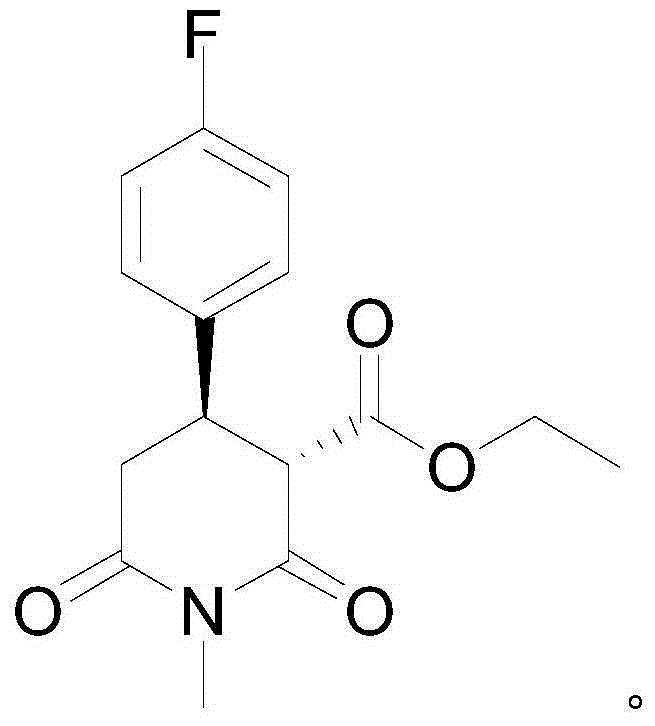
[0035] Example 1: Synthesis of (3S,4R)-1-methyl-4-p-fluorophenyl-2,6-piperidinedione-3-formic acid ethyl ester
[0036] Reaction steps: add 12mmol N-methylmalonate monoester, 10mL DMSO to the reactor, cool the reactor to -5°C, add 14mmol NaH, stir for 10min, then add chiral fluorocinnamic acid and ( 3R,4S)-Parol formed ester 10mmol. Naturally raised to room temperature until the end of the reaction.
[0037] Post-processing step: add 5% dilute hydrochloric acid aqueous solution to the reactor until the reaction system becomes acidic (pH value is about 2-3), the reaction mixture is extracted twice with ethyl acetate, the combined ethyl acetate is dried and recovered, The crude product was recrystallized from isopropanol to obtain 9 mmol of the desired product with a yield of 85%. The chemical purity is 98%, and the optical purity is 64%. Add 20% NaOH aqueous solution to the acidic aqueous layer after ethyl acetate extraction to make it alkaline, extract it twice with ethyl a...
Embodiment 2
[0038] Example 2: Synthesis of (3S,4R)-1-methyl-4-p-fluorophenyl-2,6-piperidinedione-3-formic acid ethyl ester
[0039] Reaction steps: Add 12mmol N-methylmalonic acid monoester and 10mL DMSO to the reactor, cool the reactor to -10°C, add 14mmol NaH, stir for 10min, then add chiral fluorocinnamic acid and octane 10 mmol of the ester formed by conitine was naturally raised to room temperature until the reaction was completed.
[0040] Post-processing step: add 5% dilute hydrochloric acid aqueous solution to the reactor until the reaction system is acidic (pH refers to about 1-2), the reaction mixture is extracted twice with ethyl acetate, and the combined ethyl acetate is dried and recovered. The crude product was recrystallized from isopropanol to obtain 9.5 mmol of the desired product with a yield of 89%. The chemical purity is 98%, and the optical purity is 77%. Add 20% NaOH aqueous solution to the acidic aqueous layer after ethyl acetate extraction to make it alkaline, extr...
Embodiment 3
[0041] Example 3: Synthesis of (3S,4R)-1-methyl-4-p-fluorophenyl-2,6-piperidinedione-3-formic acid ethyl ester
[0042] Reaction steps: Add 12mmol N-methylmalonic acid monoester and 10mL DMSO to the reactor, cool the reactor to -15°C, add 14mmol NaH, stir for 10min, then add chiral fluorocinnamic acid and quinol 10 mmol of the ester formed by Ning, naturally rose to room temperature until the end of the reaction.
[0043] Post-processing step: add 5% dilute hydrochloric acid aqueous solution to the reactor until the reaction system is acidic (pH refers to about 2-3), the reaction mixture is extracted twice with ethyl acetate, and the combined ethyl acetate is dried and recovered. The crude product was recrystallized from isopropanol to obtain 9.5 mmol of the desired product with a yield of 90%. The chemical purity is 98%, and the optical purity is 83%. Add 20% NaOH aqueous solution to the acidic water layer after ethyl acetate extraction to make it alkaline, extract it twice...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com