Fenofibrate delayed-release pellets, preparation method and application
A fenofibrate and pellet technology, which is applied in the field of fenofibrate pellets and preparation, can solve the problems that hinder the bioavailability of fenofibrate, the difficulty of fenofibrate delayed-release preparations, and the lack of fenofibrate. problems such as delayed-release preparations, to achieve the effect of high bioavailability, low process equipment requirements, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] The preparation of embodiment 1 loaded pill core
[0050] A prescription
[0051] Table 1:
[0052]
[0053]
[0054] *In the table, q.s. means appropriate amount.
[0055] B preparation process
[0056] Preparation of fenofibrate drug-loaded pellet core by fluidized bed powder drug loading method:
[0057] Fenofibrate bulk drug powder (Jiangsu Nhua Pharmaceutical Co., Ltd.) was micronized by a jet mill (Shanghai Huali Sofi Technology Co., Ltd., model: JGM-H100). The feed rate is 3kg / h, the working pressure is 8Mpa, and the fine powder obtained after continuous pulverization twice is measured by a laser particle size analyzer. The results showed that the drug particle size was 7.7 μm. Mix micronized fenofibrate powder and glidant evenly and add it into the feeder; use the blank pellet core as the mother core, and place it in the material tank of the tangential spray device; adjust the fluidization air volume to 100m 3 / h, the rotary speed of the turntable is...
Embodiment 2
[0058] The preparation of embodiment 2 delayed-release pellets
[0059] A prescription
[0060] Table 2:
[0061]
[0062]
[0063] *: PO: powder of copolymer of ethyl acrylate, methyl methacrylate and trimethylaminoethyl methacrylate (molar ratio 1:2:0.1) (purchased from Evonic Company)
[0064] 100: Particles of ethyl acrylate, methyl methacrylate and trimethylaminoethyl methacrylate chloride (molar ratio 1:2:0.1) copolymer (purchased from Evonic Company)
[0065] 100: particles of butyl methacrylate, dimethylaminoethyl methacrylate and methyl methacrylate (molar ratio 1:2:1) copolymer (purchased from Evonic Company)
[0066] PO: Powder of butyl methacrylate, dimethylaminoethyl methacrylate and methyl methacrylate (molar ratio 1:2:1) copolymer (purchased from Evonic Company)
[0067] B preparation process
[0068] a. Preparation of coating liquid: take respectively the plasticizer of the prescription amount shown in Table 2 and place it in a beaker, add eth...
Embodiment 3
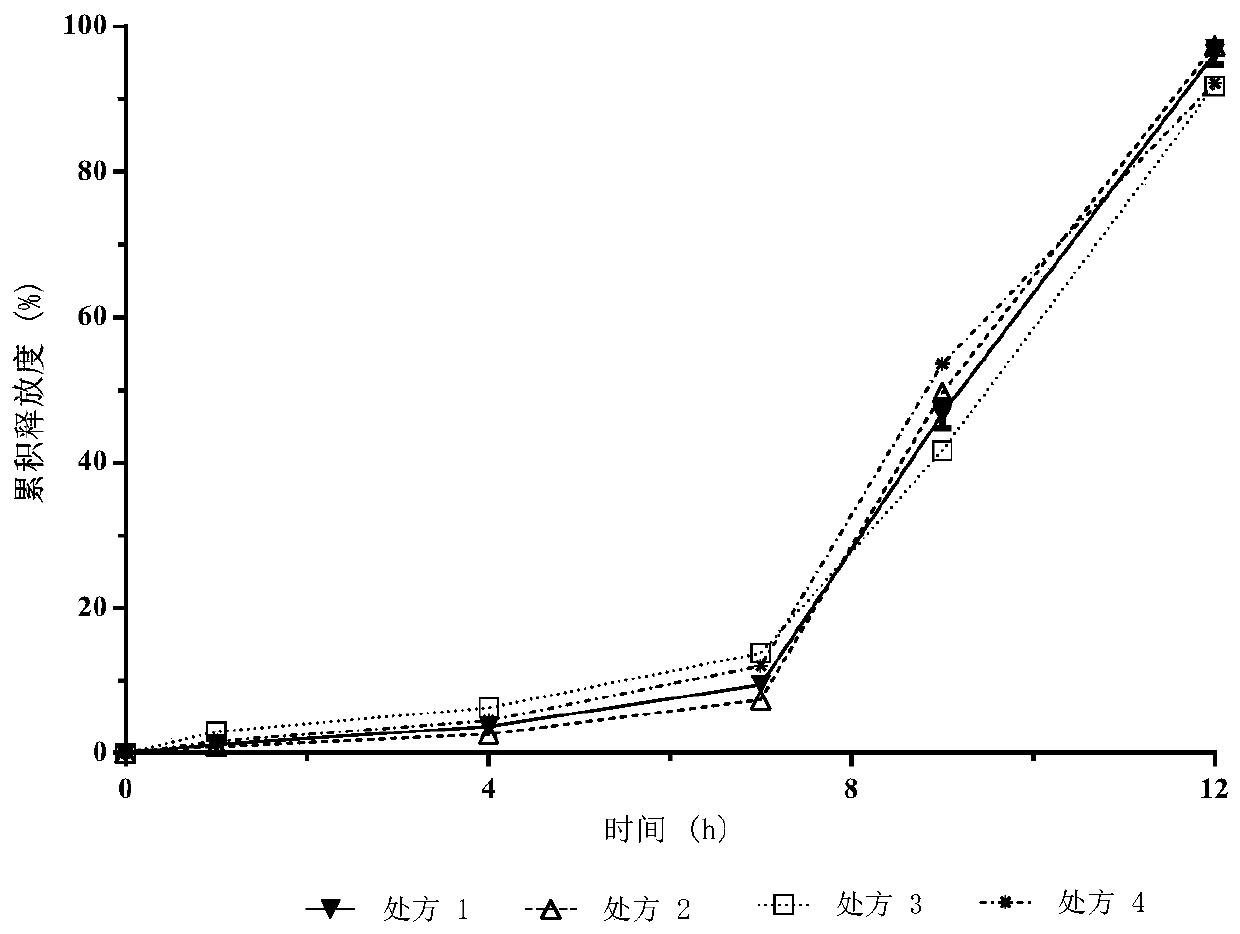
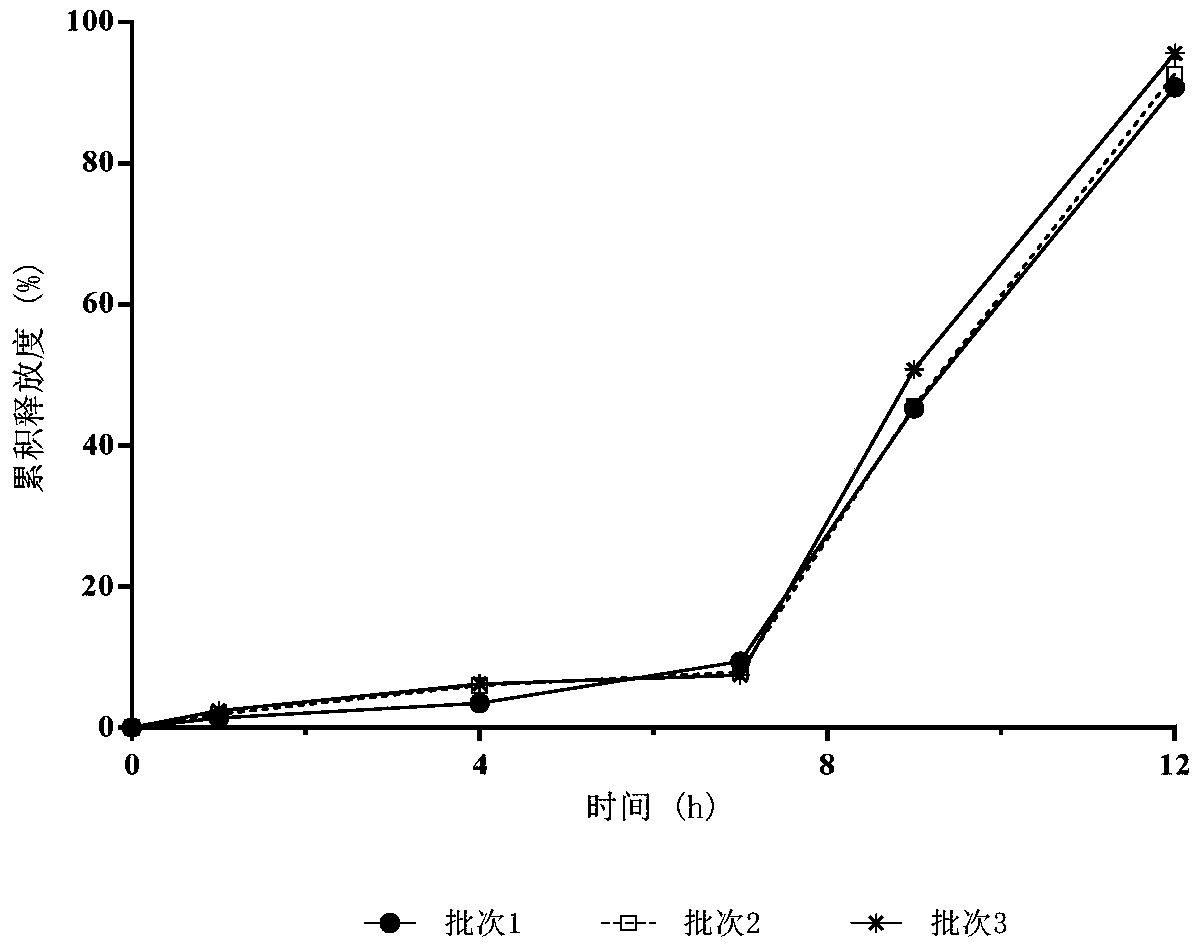
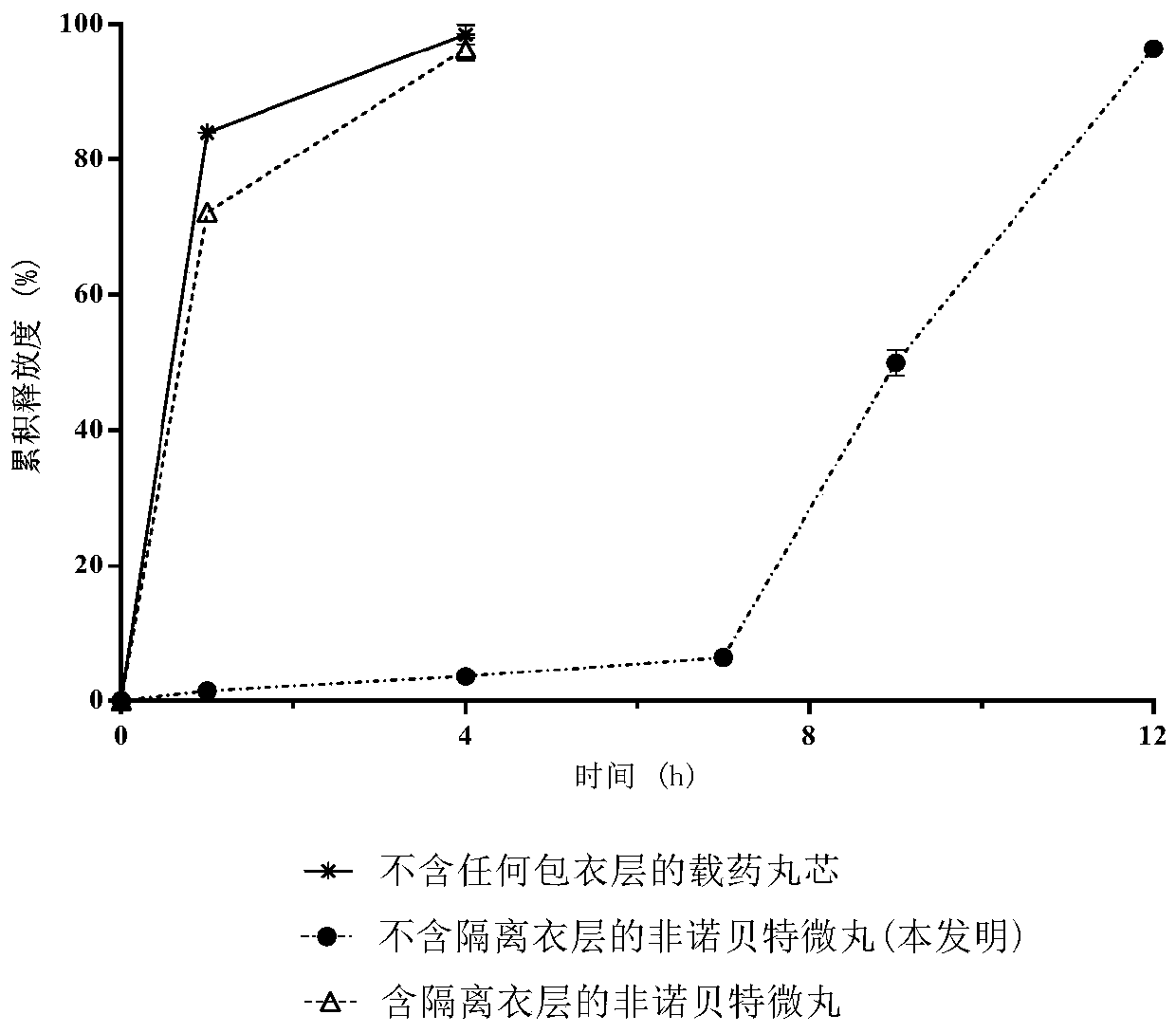
[0071] Drug cumulative release test
[0072] (i) Method for determination of release rate:
[0073] Get an appropriate amount of fenofibrate delayed-release pellets (equivalent to fenofibrate 250mg), according to the release assay method (Chinese Pharmacopoeia 2010 edition appendix XD), with pH 4.0 aqueous solution 1000mL containing sodium lauryl sulfate (SLS) As a solvent, the rotating speed is 60 revolutions per minute. Operate according to the law. Take 2mL of the solution at 1, 4, 7, 10, and 18 hours respectively, centrifuge for 1min (10000rpm), and take the supernatant as the test solution.
[0074] In addition, accurately weigh an appropriate amount of fenofibrate reference substance, add 1 mL of methanol, ultrasonically dissolve, and dilute with a pH 4.0 aqueous solution containing sodium lauryl sulfate (SLS) to a 0.125 mg / mL solution as the reference substance solution.
[0075] Take 10 μL each of the test solution and the reference solution and inject them into the l...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com