Solid catalyst used in acetone self-condensation reaction, and preparation method and application thereof
A solid catalyst, self-condensation technology, used in the preparation of organic compounds, carbon-based compounds, chemical instruments and methods, etc. Bonding, improving activity and selectivity, uniform dispersion effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
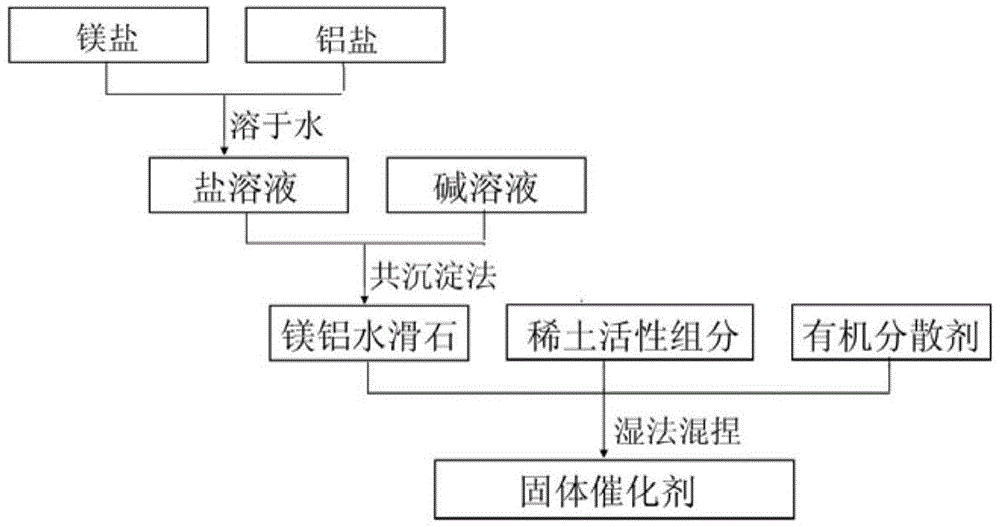
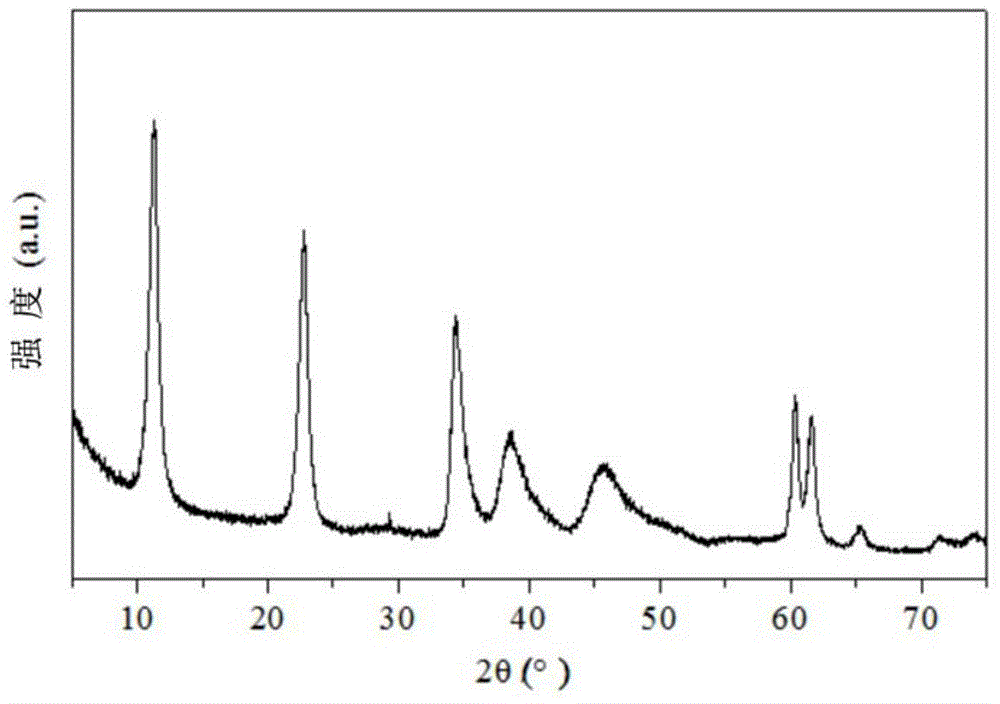
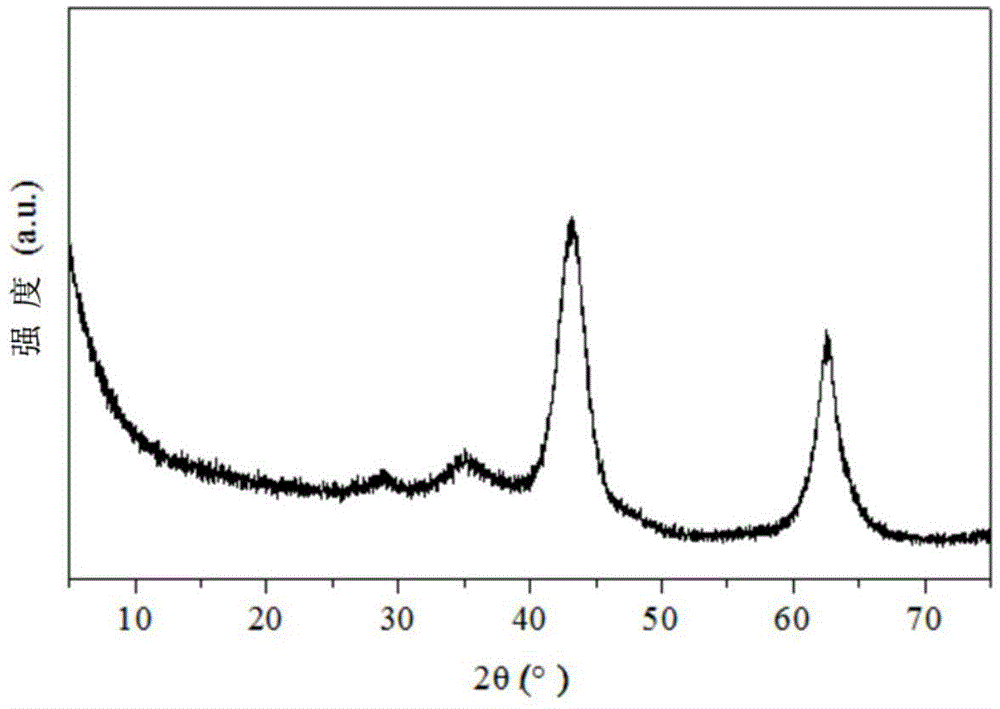
[0034] In this example, the catalyst precursor is prepared by co-precipitation method, and then the solid catalyst is prepared by wet kneading, such as figure 1 As shown, the specific process of the preparation method is as follows:
[0035] 1. Preparation of mixed salt solution:
[0036] Weigh 666.7g Mg(NO 3 ) 2 ·6H 2 O and 322.2g Al(NO 3 ) 3 9H 2 O crystals, add deionized water, stir to dissolve the salt completely, and prepare a 1000ml mixed salt solution;
[0037] 2. Preparation of alkaline solution:
[0038] Take a certain amount of sodium hydroxide, dissolve it in deionized water, and prepare a NaOH solution with a concentration of 3.8mol / L, then weigh a certain amount of sodium carbonate, dissolve it in deionized water, and prepare a NaOH solution with a concentration of 1.0mol / L. 2 CO 3 solution.
[0039] 3. Preparation of magnesium aluminum hydrotalcite:
[0040] Add 500ml deionized water into the reaction kettle, after heating to 60°C, mix salt solution an...
Embodiment 2
[0048] In this example, the catalyst precursor is prepared by the co-precipitation method, and then the solid catalyst is prepared by wet kneading. The specific process is as follows:
[0049] 1. Preparation of mixed salt solution:
[0050] Weigh 650.5g Mg(NO 3 ) 2 ·6H 2 O and 310.2g Al(NO 3 ) 3 9H 2 O crystals, add deionized water, stir to dissolve the salt completely, and prepare a 1000ml mixed salt solution;
[0051] 2. Preparation of alkaline solution:
[0052] Take a certain amount of sodium hydroxide, dissolve it in deionized water, and prepare a NaOH solution with a concentration of 3.8mol / L, then weigh a certain amount of sodium carbonate, dissolve it in deionized water, and prepare a NaOH solution with a concentration of 1.0mol / L. 2 CO 3 solution.
[0053] 3. Preparation of magnesium aluminum hydrotalcite:
[0054] Add 500ml deionized water into the reaction kettle, after heating to 65°C, mix salt solution and alkali solution (NaOH solution and NaOH solution...
Embodiment 3
[0060] In this example, the catalyst precursor is prepared by the co-precipitation method, and then the solid catalyst is prepared by wet kneading. The specific process is as follows:
[0061] 1. Preparation of mixed salt solution:
[0062] Weigh 700.4g Mg(NO 3 ) 2 ·6H 2 O and 352.1g Al(NO 3 ) 3 9H 2 O crystals, add deionized water, stir to dissolve the salt completely, and prepare a 1000ml mixed salt solution;
[0063] 2. Preparation of alkaline solution:
[0064] Take a certain amount of sodium hydroxide, dissolve it in deionized water, and prepare a NaOH solution with a concentration of 3.8mol / L, then weigh a certain amount of sodium carbonate, dissolve it in deionized water, and prepare a NaOH solution with a concentration of 1.0mol / L. 2 CO 3 solution.
[0065] 3. Preparation of magnesium aluminum hydrotalcite:
[0066] Add 500ml deionized water into the reaction kettle, after heating to 50°C, mix salt solution and alkali solution (NaOH solution and NaOH solution...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com