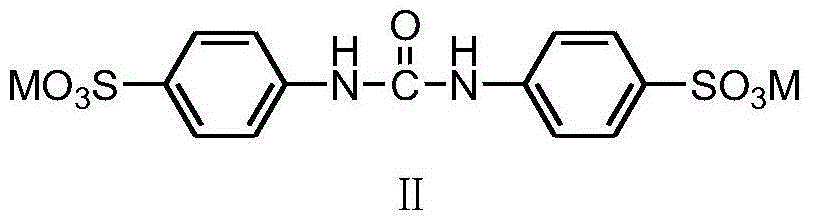Forchlorfenuron sulfonate and use thereof
A technology for the use of pyripyridine sulfonate and its use, which is applied in the field of plant growth regulators in pesticides, and can solve the problems of unreported cell division activity of pyripyridine sulfonate, unsatisfactory cell division activity, and compatibility of active components, etc. , to achieve the effects of improving the safety of action and plant absorption efficiency, improving comprehensive functions, and increasing grape particles
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
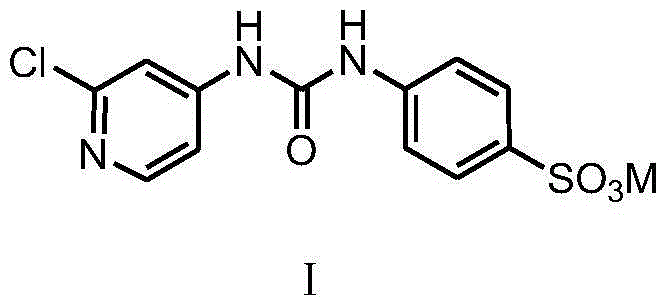
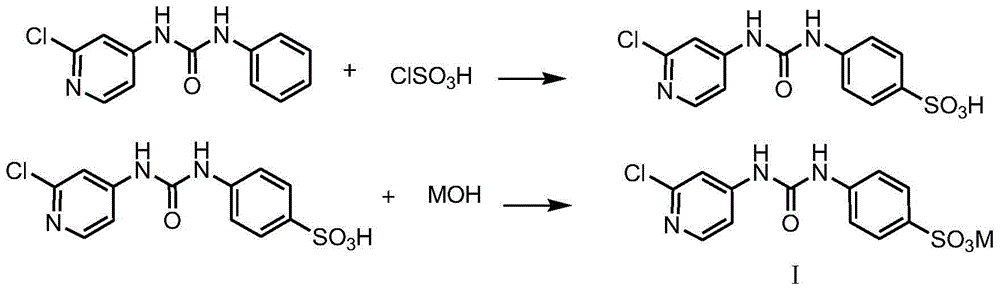
[0022] Example 1, the preparation of formula I compound
[0023] (1) The preparation of difenuron sulfonic acid potassium salt
[0024] In a 250mL three-necked flask, add 24.77g (0.1mol) fenfluramide and 100mL dichloromethane respectively, add dropwise a solution consisting of 12.82g (0.11mol) chlorosulfonic acid and 30mL dichloromethane under stirring at room temperature, and dissolve it completely. Stir and react at 20-40°C for 3 hours, and use 20% KOH solution to control the temperature below 20°C to neutralize the reaction solution to about pH=7. Remove the solvent dichloromethane by rotary evaporation, dehydrate toluene under reflux until anhydrous, filter with suction, and dry to obtain a white solid, heat the obtained solid with 150mL isopropanol to dissolve, filter out insoluble matter (inorganic salt) while hot, and cool the filtrate naturally Return to room temperature, filter with suction, wash the filter cake once with an appropriate amount of isopropanol, filter ...
example 2
[0029] Example 2, the preparation of fenpyridine sulfonate aqueous solution
[0030] (1) Preparation of 2% Potassium Difenuron Sulfonate Saline Agent
[0031] Weigh 2.0 g of potassium diuretide sulfonate, add 93 g of tap water, stir and dissolve to a transparent solution, then add 5 g of Tween-80, and stir evenly to obtain 2% potassium diuretide sulfonate salt solution.
[0032] (2) Preparation of 5% tributyramide sodium sulfonate saline solution
[0033] Weigh 5.0g of pyripuron sulfonate sodium salt, add 90g of tap water, stir to dissolve, then add 5gNP-10 (nonylphenol polyoxyethylene ether), stir and dissolve until clear and transparent to obtain 5% pyripuron sulfonate sodium brine agent.
[0034] (3) Preparation of 10% ammonium sulfonate saline solution
[0035] Weigh 10 g of ammonium tributuron sulfonate, add 80 g of tap water, stir and dissolve to a transparent solution, then add 10 g of alkyl glucoside, and stir evenly to obtain 10% ammonium dipyridouron sulfonate sal...
example 3
[0037] Example 3, cucumber cotyledon expansion test
[0038] The cucumber variety to be tested was Jinyan No. 4. After soaking the seeds, they were sown in an enamel dish with a cover filled with 0.7% agar, and cultured in a dark environment at 26°C for 72 hours. The cotyledons with uniform size were selected for use. The filter paper disc method in the determination of hormone active substances.
[0039] The specific method is: prepare the sulfonate salt of the compound of the formula I and the potassium salt of the compound of the formula II according to the method (1) of Example 2 to prepare 2% potassium salt of the sulfonate of the compound of the formula I, and then use distilled water to prepare 100 μg·mL -1 , 10μg·mL -1 aqueous solution. Dissolve fenflufenuron in an appropriate amount of ethanol, add water and 5% Tween-80 to prepare 0.5% EC, and then dilute to 100 μg·mL with distilled water -1 , 10μg·mL -1 solution. Take 0.3mL of each mother solution and drop evenl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com