Nickel phosphide catalyst containing sulphur and application of nickel phosphide catalyst
A catalyst, nickel phosphide technology, used in physical/chemical process catalysts, organic chemistry, hydrogenation to hydrocarbons, etc., can solve the problems of low activity of transition metal phosphides, not attracting attention, etc., and achieve easy quantitative preparation, improved activity, The effect of improving hydrogenation activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Preparation of Ni by Temperature Programmed Reduction of Phosphate Precursor 2 p.
[0037] 3.90 g of nickel nitrate (Ni(NO 3 ) 2 4H 2 O) and 1.77 grams of diammonium hydrogen phosphate ((NH 4 ) 2 HPO 4 ) was added to 20 mL of deionized aqueous solution, and then the pH of the solution was adjusted to 2-3 with concentrated nitric acid to obtain a clear green solution. Continue heating and stirring to evaporate water to obtain a solid product, dry at 120°C for 12 hours to dry the water, and bake at 500°C for 3 hours to obtain a phosphate precursor with a Ni / P molar ratio of 1.
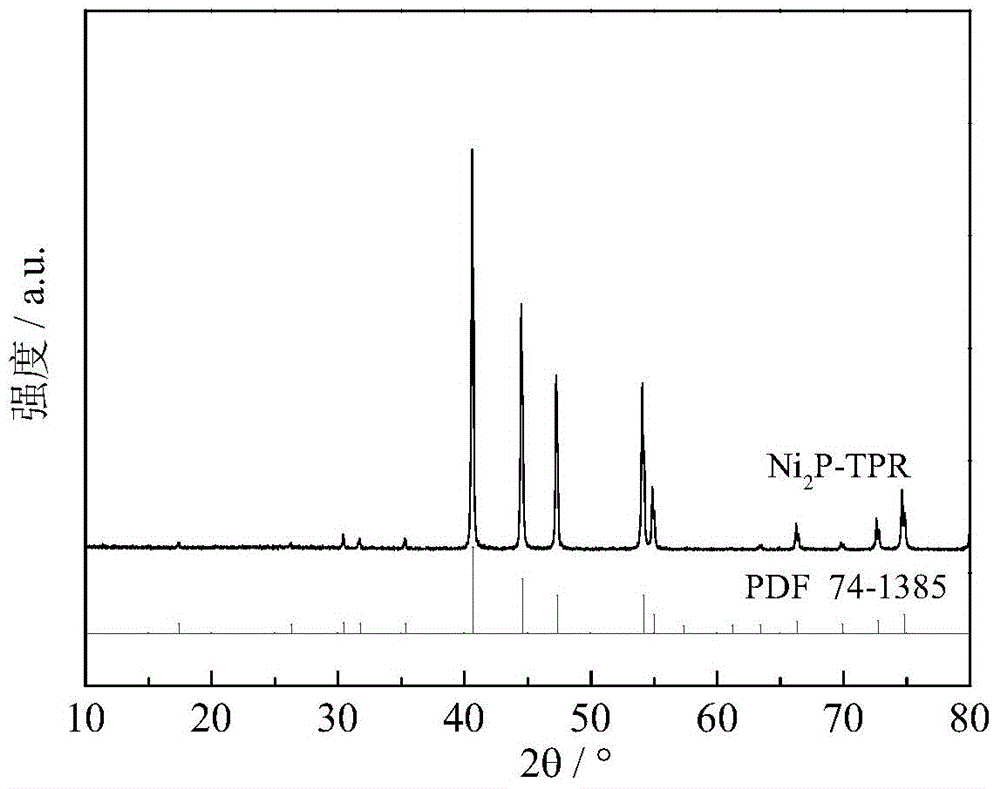
[0038] The phosphide catalyst was prepared by in-situ temperature-programmed reduction method. The temperature programming step mainly includes two steps: (1) in H 2 Under atmosphere (flow rate 150mL / min), the temperature was raised from room temperature to 120°C at 5°C / min, and kept at 120°C for 1 hour to drive off the water adsorbed by the catalyst; (2) from 120°C to 5°C / min. to 400°C, ...
Embodiment 2
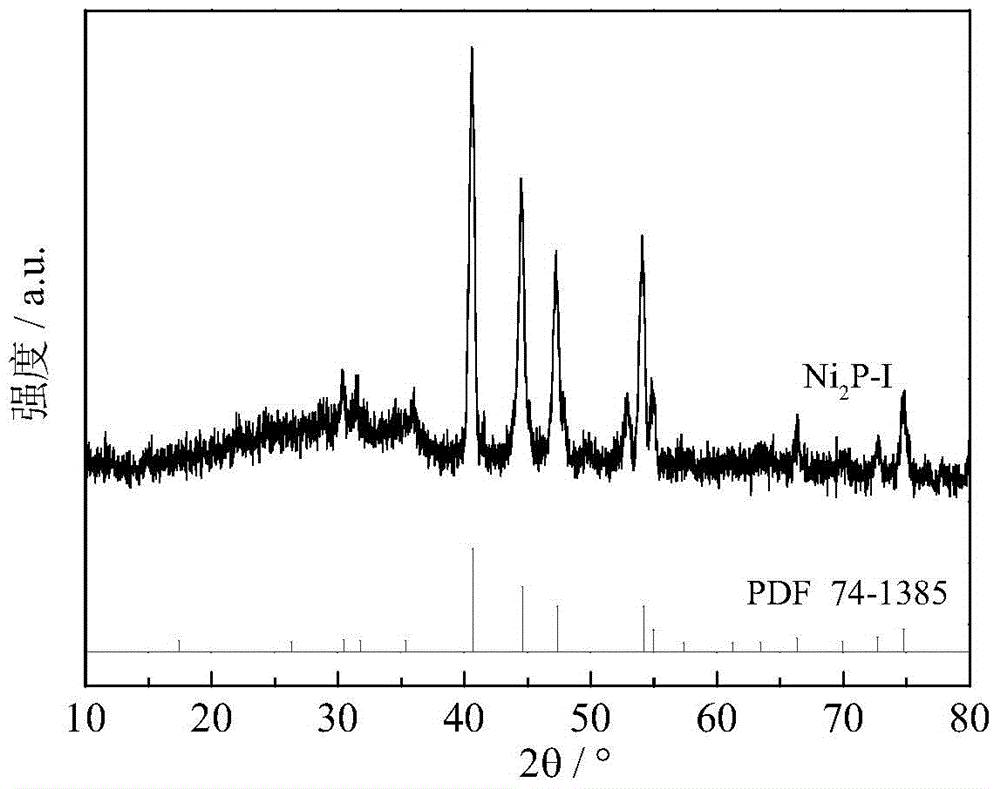
[0040] After the catalyst was prepared according to the method described in Example 1, the temperature was lowered to 340° C. for sulfidation. The used vulcanization liquid raw material is the dimethyl disulfide / decalin solution of 0.8% (mass fraction), and the vulcanization condition is: H 2 Pressure 4MPa, temperature 340°C, liquid hourly space velocity 54 hours -1 , the sulfidation treatment time is 2 hours, and the prepared catalyst is recorded as Ni 2 P-I. According to the XRD spectrum ( figure 2 ), with Ni 2 P standard spectrum comparison (PDF 74-1385), shows that the obtained solid structure is Ni 2 p. XPS spectrum ( Figure 5 ) indicates that the surface of the catalyst contains sulfur, and according to the position of the characteristic peak, the main sulfur species is S δ- (0<δ≤2). The sulfur content (in atomic percent) was 2.2%.
Embodiment 3
[0042] Preparation of Ni by Reduction of Phosphate Precursors by Hydrogen Cooled Plasma 2 P active phase, then use H under an inert or reducing gas atmosphere 2 S passivated the surface to prepare the catalyst.
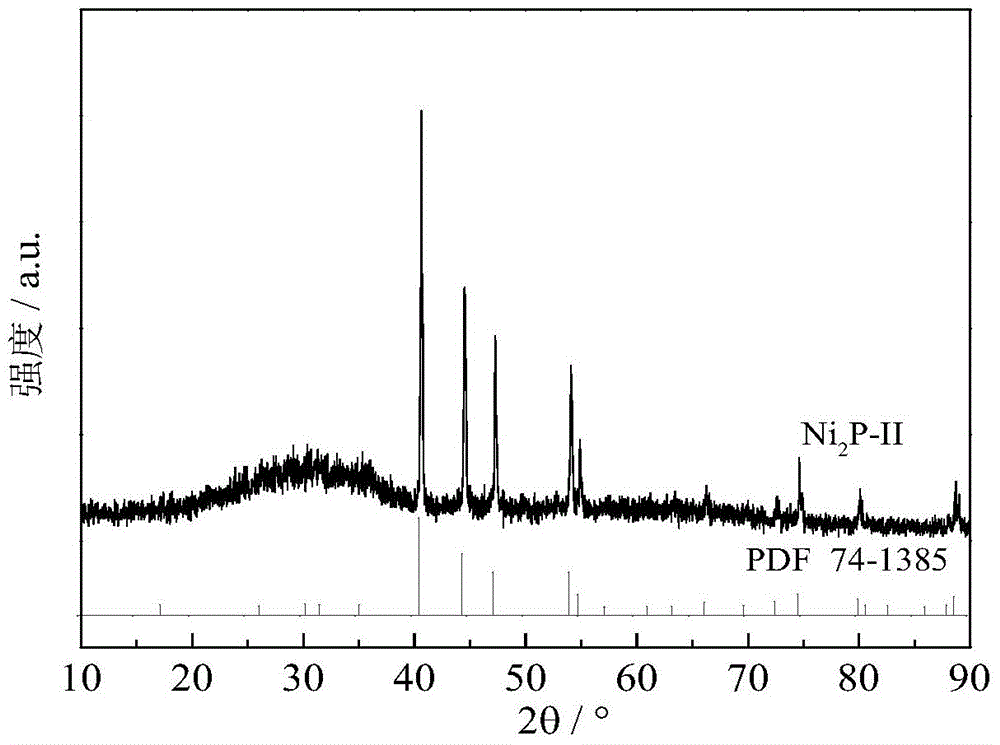
[0043] The dielectric barrier discharge (DBD) is used to generate hydrogen non-equilibrium plasma for the reduction of phosphate precursors to prepare transition metal phosphides. Take 0.8 g of the phosphate precursor in Example 1, grind, tablet, pulverize and sieve to obtain 20-40 mesh particles, which are filled in the annulus between the electrodes in the reactor. The discharge frequency is fixed at about 10kHz, and the input voltage is 70V. Atmospheric pressure, H 2 The gas velocity is 150mL / min, and the treatment time is 2 hours, and the Ni 2 p. Then pass through 10% H 2 S / H 2 The gas passivates the surface of the newly synthesized metal phosphide, and the catalyst is denoted as Ni 2 P-II. According to the XRD spectrum ( image 3 ), with Ni 2 P standar...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com