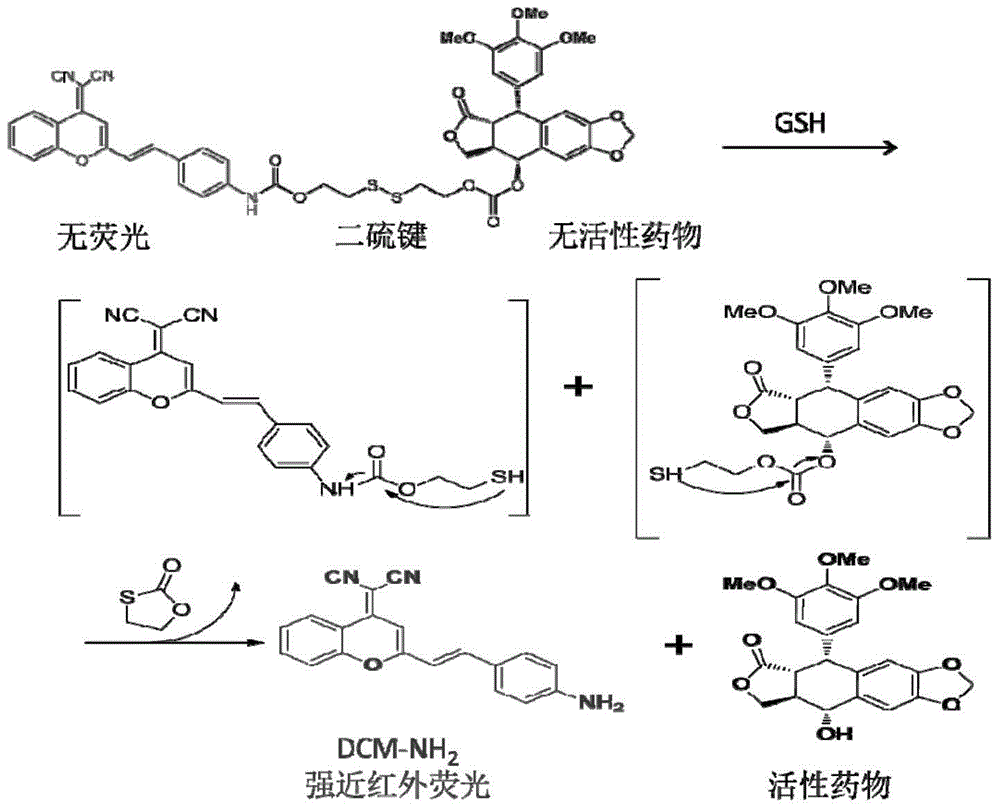
Prodrug of compound podophyllotoxin PPT with anti-tumour activity and preparation method thereof
A technology of anti-tumor activity and podophyllotoxin, applied in pharmaceutical formulations, luminescent/biological dyeing preparations, preparations for in vivo tests, etc., to achieve improved biocompatibility and less toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
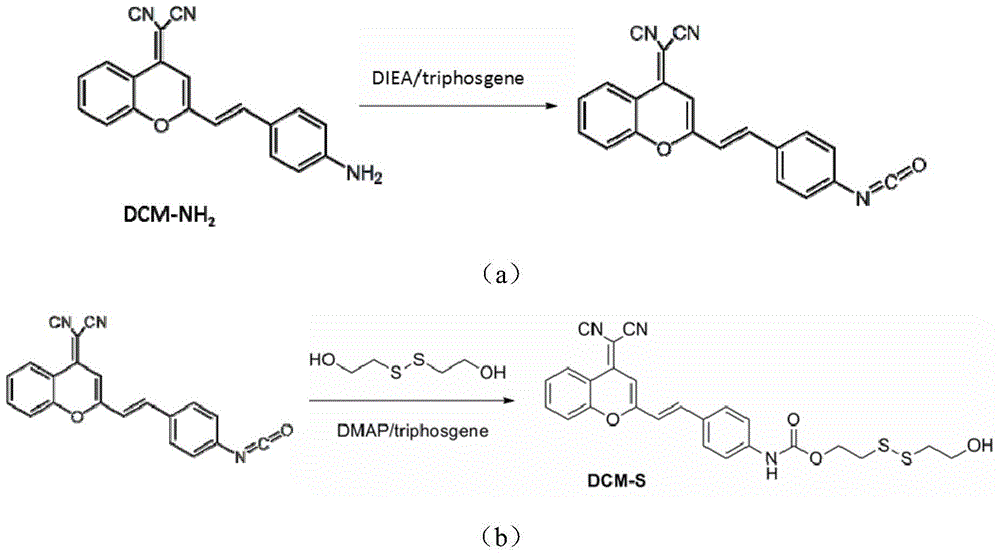
[0039] DCM-NH 2 Activation and synthesis of DCM-S:
[0040] Accurately weigh DCM-NH 2 (225mg, 0.72mmol) and triphosgene (860mg, 2.9mmol) were added to a 100mL three-necked flask containing 35mL of dry toluene, and DIEA (1.5g, 11.7mmol) was slowly added dropwise at room temperature under the protection of argon. , the above solution was heated to 111 ° C under the protection of argon for 3h reflux reaction, the activation reaction is as follows image 3 (a) shown. After the reaction, argon was blown in the solution to remove the remaining phosgene, and under argon atmosphere, 2-hydroxyethyl disulfide (1.2g, 90%, 7.2mmol) was added as a solvent with THF / DCM solution, and in DMAP Under catalysis, stirring overnight at room temperature, the addition reaction is as follows image 3 (b) shown. The solvent was removed by rotary evaporation under reduced pressure, and EA / PE (v / v, 1:1) was used as the eluent for column chromatography separation and purification to obtain 96 mg of ...
Embodiment 2
[0042] Synthesis of DCM-S-PPT:
[0043] Weigh DMAP (122mg, 1.0mmol) and vacuum dry it in a 50mL round bottom flask for 15min, then weigh podophyllotoxin (50mg, 0.1mmol) and triphosgene (20mg, 0.07mmol) into the round bottom flask, stopper Put in a round bottom flask, then add 10 mL of dry chloroform with a syringe under the condition of an ice bath and avoid light, and stir magnetically until the solution is clear. Then take the benzopyranonitrile derivative DCM-S (50mg, 0.1mmol) and dissolve it in 5mL of dry chloroform, drop it into the round bottom flask under ice bath and stir to room temperature until the solution turns light yellow, the reaction is as follows Figure 4 shown. The solvent was spin-dried and separated by column chromatography (developer DCM:EA:PE=1:0.8:1.2) to obtain 10 mg of a khaki solid with a yield of 11%. The NMR characterization results are as follows Figure 4 (b) and 4(c), indicating that DCM-S-PPT has been successfully synthesized. 1 H NMR (400...
Embodiment 3
[0045] In vitro GSH response experiment of DCM-S-PPT:
[0046] Prepare a mixed solvent of DMSO / PBS=1:1, take 2mL and add it to a cuvette, then add 2μL of DCM-S-PPT (20mM) mother solution to make the final concentration 10μM, mix well, put into the pre-adjusted in a good 37°C water bath, then add the freshly prepared reduced GSH (0.1M), and react at 37°C for 1 hour, as Figure 5 As shown in (a), the color change of the solution can be observed, and the UV-visible absorption spectrum also has a red shift, which proves that the structure of DCM-S-PPT changes and the disulfide bond may be cut.
[0047] Use high-resolution mass spectrometry (HRMS) to analyze the reaction solution of DCM-S-PPT (10 μM) and GSH (200 μM) at 37 ° C for 1 h, as Figure 5 As shown in (b), the charge-to-mass ratio of 310.1 (DCM-NH 2 ) and 413.2 (PPT) molecular ion peaks, once again proved that the sulfhydryl group on the GSH cut off the disulfide bond in DCM-S-PPT, and then by removing a sulfur-containin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com