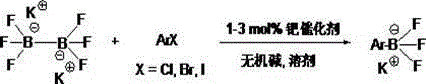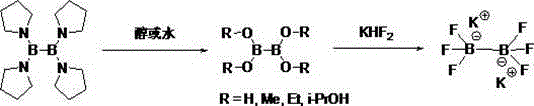Novel method for synthesizing potassium aryl trifluoroborate
A technology of potassium aryl trifluoroborate and potassium trifluoroborate, which is applied in the field of synthesizing potassium aryl trifluoroborate, can solve the problems such as no public data reports and the like, and achieve the effect of direct and efficient synthesis and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Synthesis of potassium trifluoroborate biborate:
[0025] Under nitrogen protection, tetrakis(tetrahydropyrrole) biboron (0.15 mol) and 18-20 equivalents of absolute ethanol were added into a three-neck flask equipped with dropping and reflux devices, and the temperature was raised to reflux for reaction. After refluxing for about 1-2 hours, start to replace it with an atmospheric distillation device to distill out the tetrahydropyrrole generated during the reaction. At this time, the ethanol solvent will also be taken out at the same time. During the whole distillation process, ethanol needs to be replenished from the dropping funnel . When the solvent to be distilled out no longer contains tetrahydropyrrole, get the reaction liquid gas phase detection to confirm that the reaction is complete, after cooling this time, obtain 25.9 grams of colorless liquid after rectification under reduced pressure, HNMR and GC-MS confirm that it is Tetra(B) Oxygen) biboron. Add 120 m...
Embodiment 2
[0027] Synthesis of potassium trifluoroborate biborate:
[0028] Under nitrogen protection, tetrakis(tetrahydropyrrole) biboron (0.1 mole) and 50-60 times deionized water were added into a three-neck flask equipped with dropping and reflux devices, and the temperature was raised to 100° C. for reflux reaction. After refluxing for 1.5 hours, replace it with an atmospheric distillation device, and distill out the tetrahydropyrrole generated during the reaction. When the tetrahydropyrrole is no longer used for distillation, cool the reaction solution to room temperature. At this time, solids are precipitated. After filtering and drying, Obtain 8.5 grams of white solid tetrahydroxydiboron, the HNMR purity is more than 98%, consistent with the literature. Add 45 ml of methanol, stir at room temperature until completely dissolved (about 10-15 minutes), then add KHF 2 Aqueous solution (0.38 mol) was stirred at room temperature for 1 hour. The system was allowed to stand still and se...
Embodiment 3
[0030] Synthesis of Potassium Phenylboronic Acid Trifluoroborate:
[0031] Under the protection of nitrogen, in a 100 ml three-necked flask, add potassium trifluoroborate (0.022 mol), bromobenzene (0.02 mol), 1M potassium carbonate solution (0.04 mol) and 65 ml of ethanol into a 100 ml three-necked flask, stir well, plug in Air tube to the liquid surface of the solution, nitrogen bubbling deoxygenation about 10-20 minutes. Subsequently, 1.0% mole of the prepared palladium catalyst (phenylethylamine, palladium acetate, potassium tert-butoxide and X-Phos is obtained after the reaction, preparation method reference: J.Am.Chem.Soc., 2010,132, 17701) nitrogen join under protection, 80 o C was reacted overnight. After the detection reaction was completed, diatomaceous earth was filtered. The filtrate was spin-dried under reduced pressure and high vacuum, 50 ml of acetone was added, the insoluble solids were filtered off, the filtrate was spin-dried again (if it could not be s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com