Immobilized maleic acid cis-trans isomerase and preparation method and application thereof
A technology of cis-trans isomerization and immobilization of enzymes, applied in the fields of enzymology, enzyme engineering, and biochemical industry, can solve the problems of difficulty in development and application, reduced activity, high price, etc., to reduce the complexity of operation and reduce the amount of enzyme. Loss, production cost saving effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1R5
[0051] Example 1 Construction of R5 short peptide and maleic acid cis-trans isomerase fusion protein engineering bacteria
[0052]Primer F: 5'-CCATATGTCCTCCAAGAAATCGGGATCCTACTCGGGATCCAAGGGTTCCAAGCGTCGCATCTTGATGAGCAACCACTACCGCATCGGCCAGATC-3' and primer R: 5'-GCTGTCCACCAGTCATGCTAGCCATATGTATATCTCC-3' were designed according to the R5 short peptide gene sequence shown in SEQ ID NO.1.
[0053] Using primer F and primer R as primers, the recombinant plasmid pET24a-maiA carrying the gene encoding maleic acid cis-trans isomerase [Wang Ya, Cui Wenjing, Zhou Li, Liu Zhongmei, Zhou Zhemin. Serratia marcescens Malay Expression, purification and enzymatic properties of acid cis-trans isomerase [J]. Journal of Food and Biotechnology, Vol. 33, No. 11, 2014, 1204-1209] as template, PCR reverse amplification, PCR conditions: 94 ℃ pre-denaturation 5min; 98°C denaturation for 10s, 68°C extension for 6min 30s, 30 cycles; 72°C for 10min.
[0054] After the PCR product was purified, it was digeste...
Embodiment 2
[0055] Induced expression of embodiment 2 fusion protein
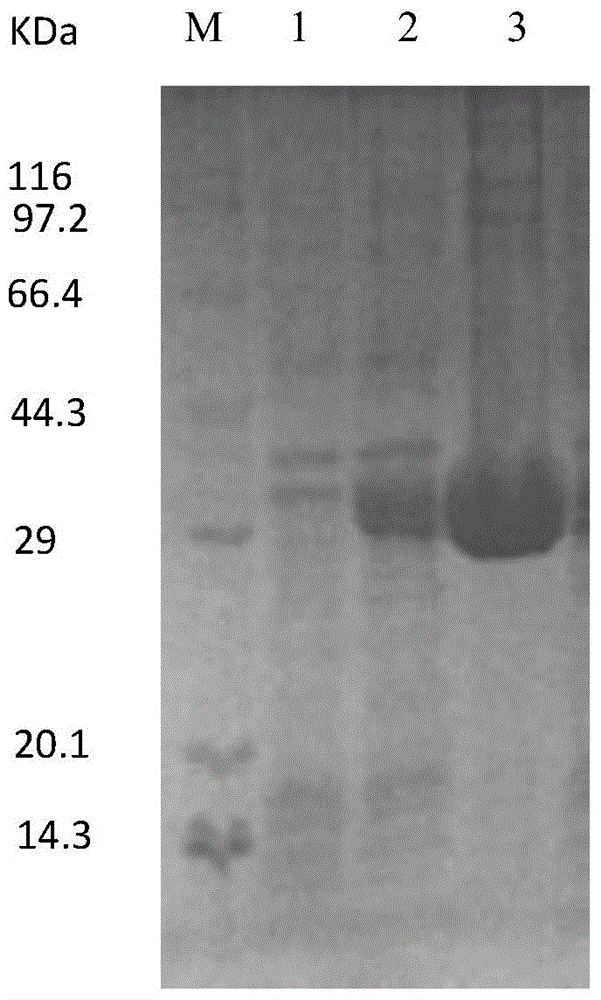
[0056] The pET24a-R5-MaiA plasmid was transformed into E.coli BL21 to obtain the E.coli BL21 / pET24a-R5-MaiA recombinant strain. E.coli BL21 / pET24a-R5-MaiA recombinant strain was cultured in LB medium containing kanapenicillin at 37°C for 8 hours, and 0.3mL culture solution was put into 30mL LB medium and cultivated to OD at 37°C 600 When the concentration was 0.6, IPTG was added to a final concentration of 0.2 mM, and the bacterial cells were collected by centrifugation after induction at 20°C for 20 h, the cell wall was broken by ultrasonic, and electrophoresed by SDS-PAGE ( figure 2 ) obtained a 29kDa size band, indicating that the fusion enzyme was successfully expressed in Escherichia coli. The specific enzyme activity of the purified recombinant enzyme R5-MaiA was 42U / ml, and that of the original enzyme MaiA was 48U / ml. The activity of the recombinant enzyme R5-MaiA did not decrease significantly after fusion, w...
Embodiment 3
[0057] Embodiment 3 Preparation of immobilized maleate cis-trans isomerase
[0058] Take the fermentation broth of the fusion engineered bacteria and centrifuge to obtain the cells, wash twice with buffer, and concentrate the cells to OD 600 =30-40, after ultrasonic crushing of the bacteria, centrifuge at 12000rpm for 10min, slowly add ammonium sulfate powder to the broken supernatant and keep stirring until the saturation reaches 60%, continue stirring for 30min, and slowly add glutaraldehyde to the final concentration of 0.05%-0.2% (v / v) and stirring continuously at room temperature for 1 h to obtain cross-linked enzyme aggregate particles, and the cross-linked enzyme aggregates obtained by centrifugation were washed 3 times with buffer, and the cross-linked enzyme aggregates and Mix the silicon reaction solution and methyl orthosilicate (ratio: 1:8:1) and stir vigorously for 30s, let stand for 5min, centrifuge at 5000rpm for 5min, collect the immobilized enzyme, and wash wi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com