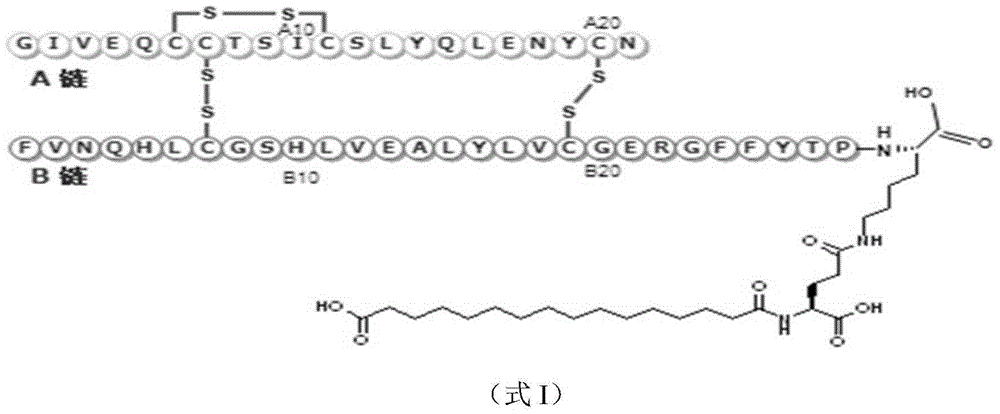
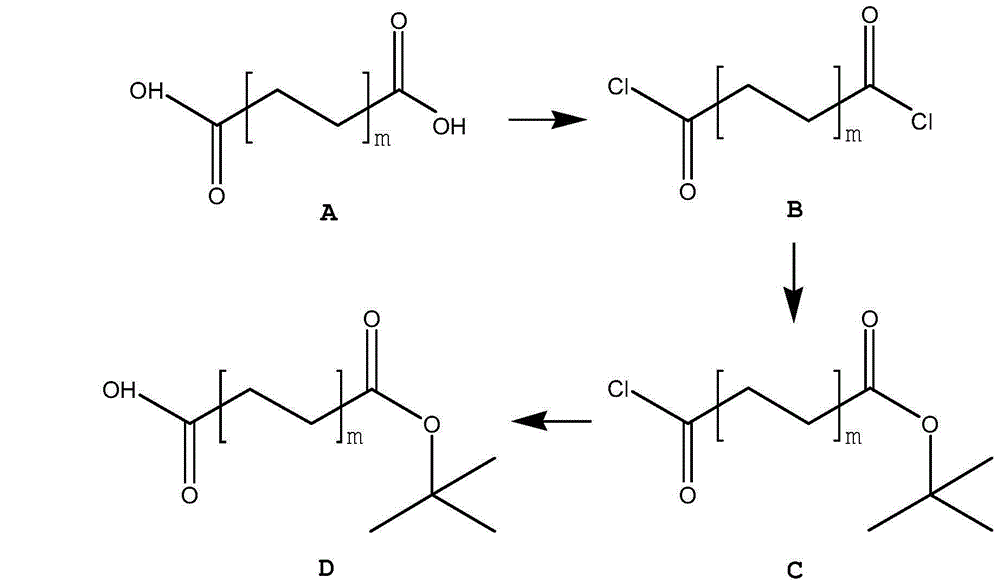
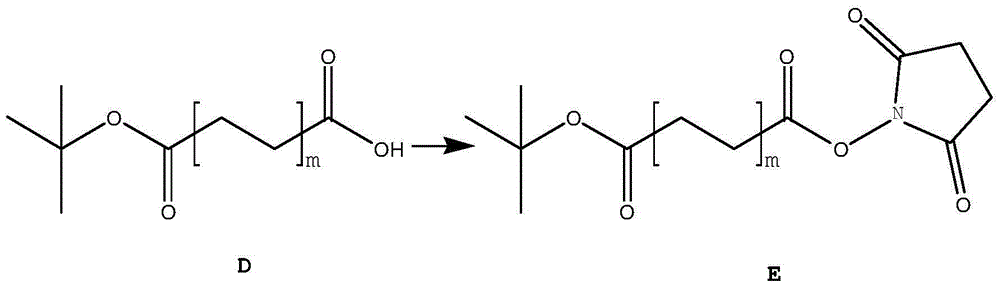
Preparation method and application of fatty dioic acid derivative
A fatty diacid and derivative technology, applied in the field of biomedicine, can solve the problems of high impurities, decomposition of insulin peptide chain, and unsuitable for industrial scale-up production.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0086] 1. Preparation of mono-tert-butyl hexadecanedioic acid
[0087]
[0088] Hexadecanedioic acid (20.0 g, 69.8 mmol) was suspended in thionyl chloride (100 ml, 1376.8 mmol), heated to reflux at 80° C. for 3 hours, and excess thionyl chloride was distilled off under reduced pressure. Add dichloromethane (200ml) to dissolve, add tert-butanol (7.34ml, 76.81mmol) and pyridine (5.9ml, 73.32mmol), add pyridine within 2 hours, and react at 25°C for 3h. The solvent was evaporated to dryness under reduced pressure, and 600ml of DMF / water mixed solvent precooled to 0-4°C was added (DMF and water were mixed in a volume ratio of 1:2), and the precipitate was collected by filtration. Vacuum dry to constant weight, add dichloromethane (300ml) to resuspend, collect the filtrate and evaporate the solvent under reduced pressure, add petroleum ether (400ml) to resuspend, collect the filtrate and evaporate to dryness under reduced pressure, add n-heptane (80ml) for recrystallization , th...
Embodiment 2
[0112] 1. Preparation of tert-butyl tetradecanedioate
[0113]
[0114] Tetradecanedioic acid (20.0g, 77.4mmol) was suspended in dichloromethane (200ml), and catalytic equivalents of DMF (89.8μl, 1.16mmol) and thionyl chloride (33.73ml, 464.4mmol) were added respectively, at 25°C Stir overnight until dissolved. The solvent was distilled off under reduced pressure, dichloromethane (200ml) was added to dissolve, tert-butanol (8.07ml, 84.49mmol) and pyridine (6.5ml, 80.65mmol) were added, pyridine was added within 2 hours, and reacted at 20°C for 3h. The solvent was evaporated to dryness under reduced pressure, and 600ml of DMF / water mixed solvent precooled to 0-4°C was added (DMF and water were mixed in a ratio of 1:3 by volume), and the precipitate was collected by filtration. Vacuum dry to constant weight, add dichloromethane (300ml) to resuspend, collect the filtrate and evaporate the solvent under reduced pressure, add petroleum ether (400ml) to resuspend, collect the fi...
Embodiment 3
[0134] 1. Preparation of mono-tert-butyl octadecanedioate
[0135]
[0136] Suspend octadecanedioic acid (20.0g, 63.6mmol) in dichloromethane (200ml), add catalytic equivalents of DMF (73.8μl, 0.95mmol) and thionyl chloride (27.72ml, 381.6mmol) respectively, and stir at room temperature Leave overnight until dissolved. The solvent was distilled off under reduced pressure, dichloromethane (200ml) was added to dissolve, tert-butanol (6.63ml, 69.43mmol) and pyridine (5.34ml, 66.27mmol) were added. Pyridine was added within 2 hours and reacted at 25°C for 3h. The solvent was evaporated to dryness under reduced pressure, and 600ml of DMF / water mixed solvent precooled to 0-4°C was added (DMF and water were mixed in a volume ratio of 1:2), and the precipitate was collected by filtration. Vacuum dry to constant weight, add dichloromethane (200ml) to resuspend, collect the filtrate and evaporate the solvent under reduced pressure, add petroleum ether (400ml) to resuspend, collect t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com