A kind of vitamin B prepared from malic acid 6 Methods
A vitamin and malic acid technology, applied in the field of pharmaceutical biochemical industry, can solve the problems of long production cycle, heavy color of products, environmental pollution, etc., and achieve the effects of low cost, easy handling, and easy metabolism and decomposition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Example 1: Vitamin B 6 (I) Preparation
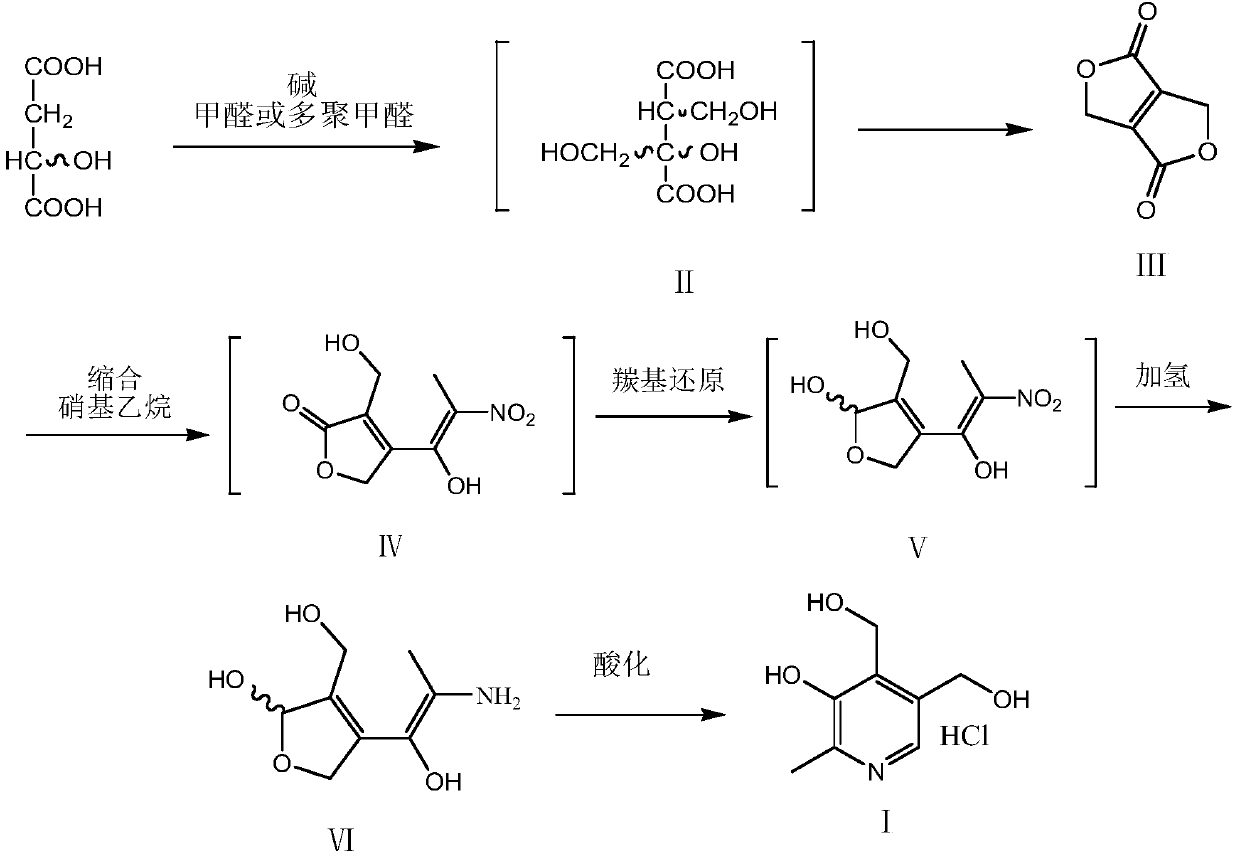
[0041] Step (1): Preparation of 1H,4H-dihydrofuro[3,4-c]dihydrofuran-1,4-dione Ⅲ
[0042] Add 100 grams of toluene, 10 grams of water, 13.4 grams (0.1 mole) of D, L-malic acid, 0.85 grams of piperidine into a 500 ml four-neck flask equipped with stirring, a thermometer and a water separator, heat up to 50 ° C, drop Add 30 g of 30% aqueous formaldehyde solution, dropwise, and react at 50° C. for 5 hours. Add 3.0 grams of p-toluenesulfonic acid, reflux with azeotropic water until the water is completely separated, cool to 20 ° C, filter to remove piperidine-p-toluenesulfonate, wash the filter cake twice with toluene (using 20 grams of toluene ), reclaiming toluene to obtain 14.1 grams of oil compound III, with a purity of 98.6% (GC), and a pure yield of 99.3%, which was directly used in the next step.
[0043] Step (2): Vitamin B 6 (I) Preparation
[0044] Add 80 grams of tetrahydrofuran, 8.3 grams (0.11 moles) of nitroethane,...
Embodiment 2
[0045] Example 2: Vitamin B 6 (I) Preparation
[0046] Step (1): Preparation of 1H,4H-dihydrofuro[3,4-c]dihydrofuran-1,4-dione Ⅲ
[0047] Add 100 grams of toluene, 15 grams of water, 13.4 grams (0.1 moles) of D, L-malic acid, 0.85 grams of piperidine into a 500 ml four-neck flask equipped with stirring, a thermometer and a water separator, heat up to 50 ° C, drop Add 30 g of 30% aqueous formaldehyde solution, dropwise, and react at 50° C. for 5 hours. Add 1.5 grams of 98% concentrated sulfuric acid, reflux with azeotropic water until the water is completely separated, cool to 20°C, filter to remove the piperidine sulfate salt, wash the filter cake twice with toluene (using 20 grams of toluene), and recycle the toluene to obtain an oily substance Compound III was 13.8 g, with a purity of 98.5% (GC), and a pure yield of 97.1%, which was directly used in the next step.
[0048] Step (2): Vitamin B 6 (I) Preparation
[0049] Add 80 grams of tetrahydrofuran, 8.3 grams (0.11 mo...
Embodiment 3
[0050] Example 3: Vitamin B 6 (I) Preparation
[0051] Step (1): Preparation of 1H,4H-dihydrofuro[3,4-c]dihydrofuran-1,4-dione Ⅲ
[0052] Add 120 grams of toluene, 10 grams of water, 13.4 grams (0.1 moles) of L-malic acid, and 1.5 grams of tri-n-butylamine into a 500 milliliter four-neck flask equipped with stirring, a thermometer and a water separator. Add 30 grams of 30% formaldehyde aqueous solution, drop it, and react at 40-45° C. for 5 hours. Add 3.5 grams of p-toluenesulfonic acid, reflux with azeotropic water until the water is completely separated, cool to 20 ° C, filter to remove tri-n-butylamine p-toluenesulfonate, wash the filter cake twice with toluene (using 20 grams toluene), reclaiming toluene to obtain 13.9 grams of oil compound III, with a purity of 98.8% (GC), and a pure yield of 98.1%, which was directly used in the next step.
[0053] Step (2): Vitamin B 6 (I) Preparation
[0054] Add 80 grams of tetrahydrofuran, 8.3 grams (0.11 moles) of nitroethane, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of polymerization | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com