Synthetic method for artificially coupling antigen through aflatoxin B1-carrier proteins
A technology of aflatoxin and carrier protein, which is applied in the field of synthesis of aflatoxin B1-carrier protein artificially coupled antigen by a two-step method, which can solve the problem of low purity requirements of the sample to be tested, the inability to achieve trace detection, and the limitation Problems such as popularization and application, to achieve the effect of easy popularization and application, mild coupling reaction and good immunogenicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
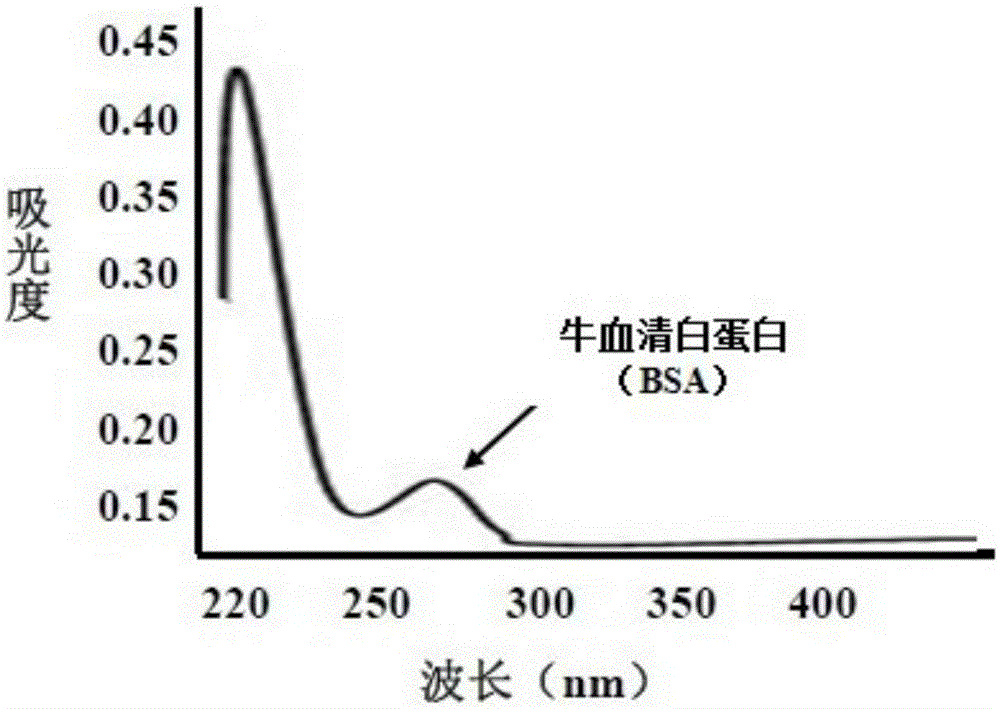
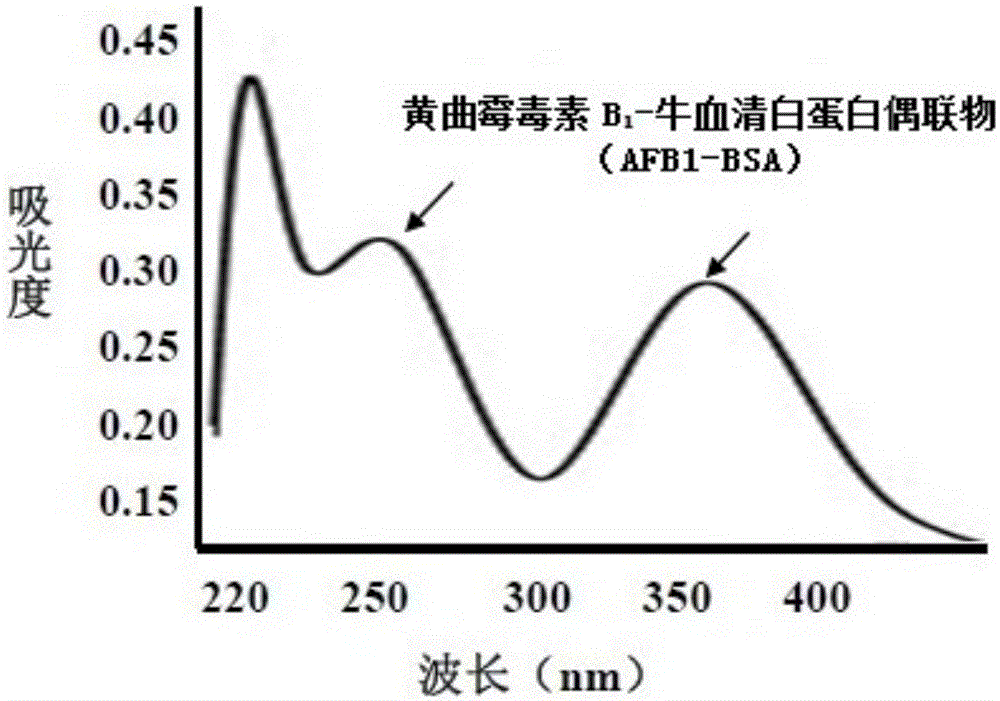
[0035] Example 1: Aflatoxin B 1 - Preparation and identification of bovine serum albumin (BSA) artificial conjugated antigen
[0036] 1. Weigh 2mg of aflatoxin B 1 Dissolve the standard product in 4ml of solvent with a volume ratio of methanol and pyridine of 1:1, and add 5m oximating agent carboxymethylhydroxylamine hemihydrochloride (CMO); after stirring for 2 hours at 70°C, place the resulting product in a fume hood Naturally evaporate to dryness; add 1ml of distilled water to dissolve, adjust the pH to 8.0 with sodium hydroxide (1mol / L), and extract unreacted AFB in the system three times with 5ml of benzene 1 , hydrochloric acid (0.2mol / L) to adjust the pH to 3.0, and finally use 5ml of ethyl acetate to extract the precipitate three times and dry it naturally under the fume hood. The obtained dry product is AFB 1 O; the resulting AFB 1 O was dissolved in 2ml of dimethylformamide (DMF), and the activation reagents, namely 8.9mg of dicyclohexylcarbodiimide (DCC) and 5.0m...
Embodiment 2
[0038] Example 2: Aflatoxin B 1 - Preparation and identification of artificial conjugated antigen from ovalbumin (OVA)
[0039] 1. Weigh 2mg of aflatoxin B 1 The standard product was dissolved in 4ml of methanol and pyridine in a solvent with a volume ratio of 1:1, and 5mg of the oximating agent carboxymethylhydroxylamine hemihydrochloride (CMO) was added, stirred at 70°C for 2 hours, and the resulting product was placed in a ventilated Evaporate naturally under the cabinet; add 1ml of distilled water to dissolve, adjust the pH to 8.0 with sodium hydroxide (1mol / L), and extract the unreacted AFB in the system three times with 5ml of benzene 1 , 0.2mol / L hydrochloric acid to adjust the pH to 3.0, and finally use 5ml ethyl acetate to extract the precipitate three times and dry it naturally under the fume hood. The obtained dry product is AFB 1 O; the resulting AFB 1 O was dissolved in 2ml of dimethylformamide (DMF), and after adding the activating reagent, that is, 8.9mg of D...
Embodiment 3
[0040] Example 3: Anti-aflatoxin B 1 Preparation and identification of polyclonal antibodies
[0041] 1. Animal experiment: the prepared aflatoxin B 1 -BSA artificially conjugated antigen (4mg / ml) was diluted with phosphate buffered saline (PBS, 0.01M, pH=7.4), mixed with an equal volume of Freund's complete adjuvant, fully emulsified, and then immunized with Balb / C mice (6-8 weeks old), multiple subcutaneous injections on the back, 100 μg protein per mouse, two weeks later, emulsify the artificial antigen with Freund’s incomplete adjuvant, and perform secondary immunization, 100 μg protein per child, two weeks later, use it with The third immunization was carried out with the same dose as the second immunization, and blood was collected from the tail of the mice 7 days after the third immunization to prepare antiserum.
[0042] 2. Indirect ELISA detection antibody titer test: to determine whether the antibody produced by the animal body is specific for aflatoxin B 1 Aflato...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com