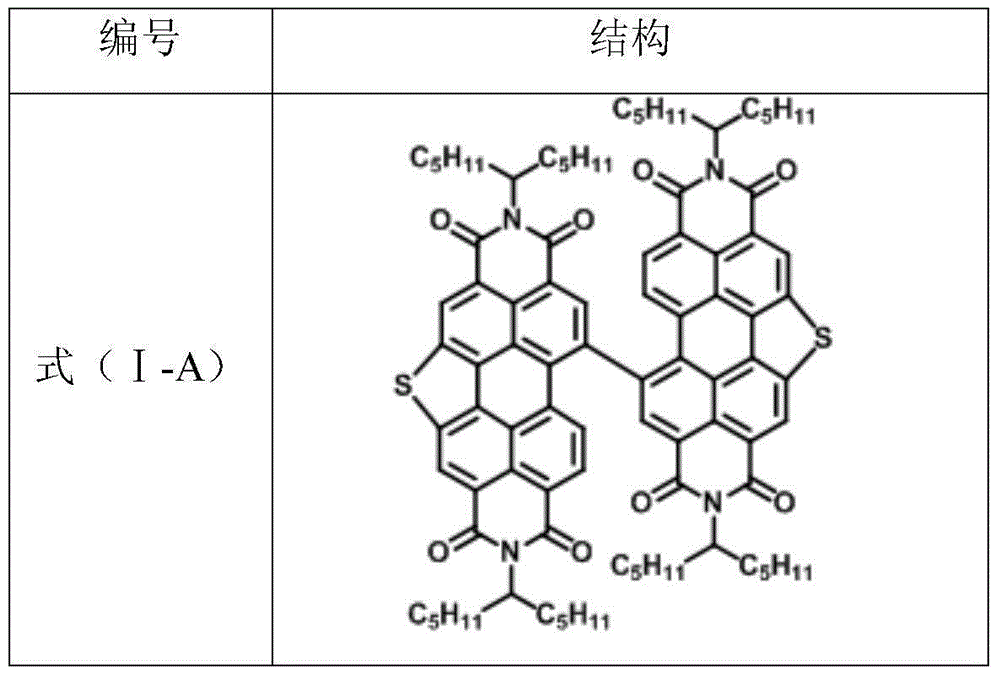
Novel heterocycle perylene imide dimer compound as well as preparation method and application thereof
A dimer perylene imide, perylene imide technology, applied in organic chemistry, semiconductor/solid-state device manufacturing, photovoltaic power generation, etc., can solve the problems of few derivative products and low yield of perylene diimide derivatives
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
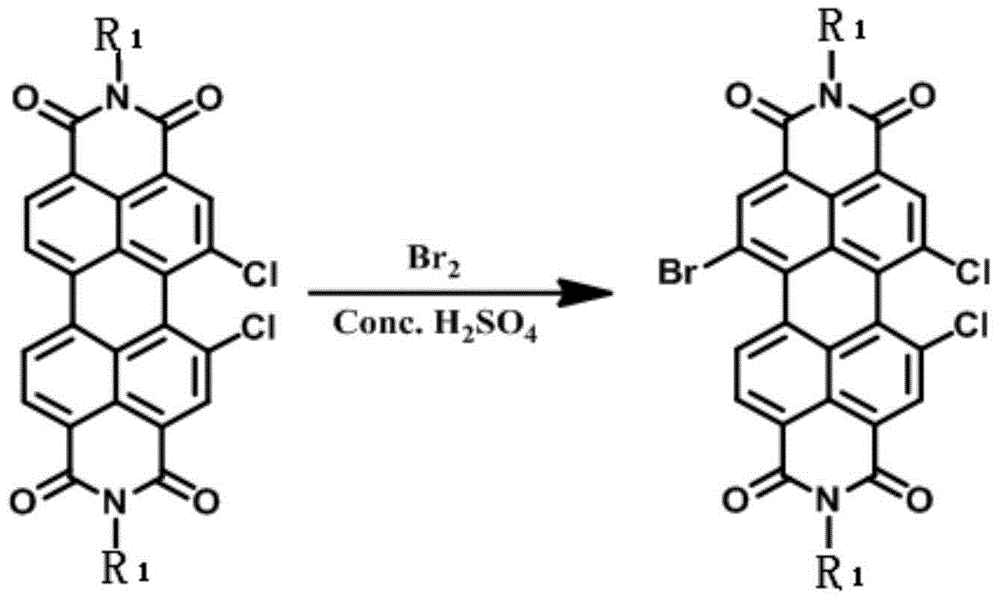
[0050] (1) Preparation of intermediate compound formula (Ⅲ-A):
[0051] Add 1,12-dichloro-peryleneimide (1g, 1.3mmol) and 60ml mass concentration of 98% concentrated sulfuric acid with a structure shown in formula (II) in a 100ml two-necked bottle, after mixing evenly, add liquid bromine (18.66g, 0.116mol), stirred and reacted at room temperature for two days, poured into 1000ml saturated aqueous sodium sulfite solution after the reaction was completed, collected the precipitate by suction filtration, washed successively with saturated aqueous sodium sulfite solution and water, dried, purified by silica gel column, dichloro Using methane / petroleum ether as a developer, the intermediate compound (III-A) (991 mg) was obtained as an orange-red solid powder with a yield of 90.1%.
[0052]
[0053] (2) Preparation of intermediate compound formula (IV-A):
[0054] In a 100ml two-necked flask, add the compound of formula (Ⅲ-A) (423mg, 0.50mmol), copper powder (<100nmparticlesize,...
Embodiment 2
[0063] (1) Preparation of intermediate compound formula (Ⅲ-A): the method is the same as in Example 1;
[0064] (2) Preparation of intermediate compound formula (IV-A): the method is the same as in Example 1;
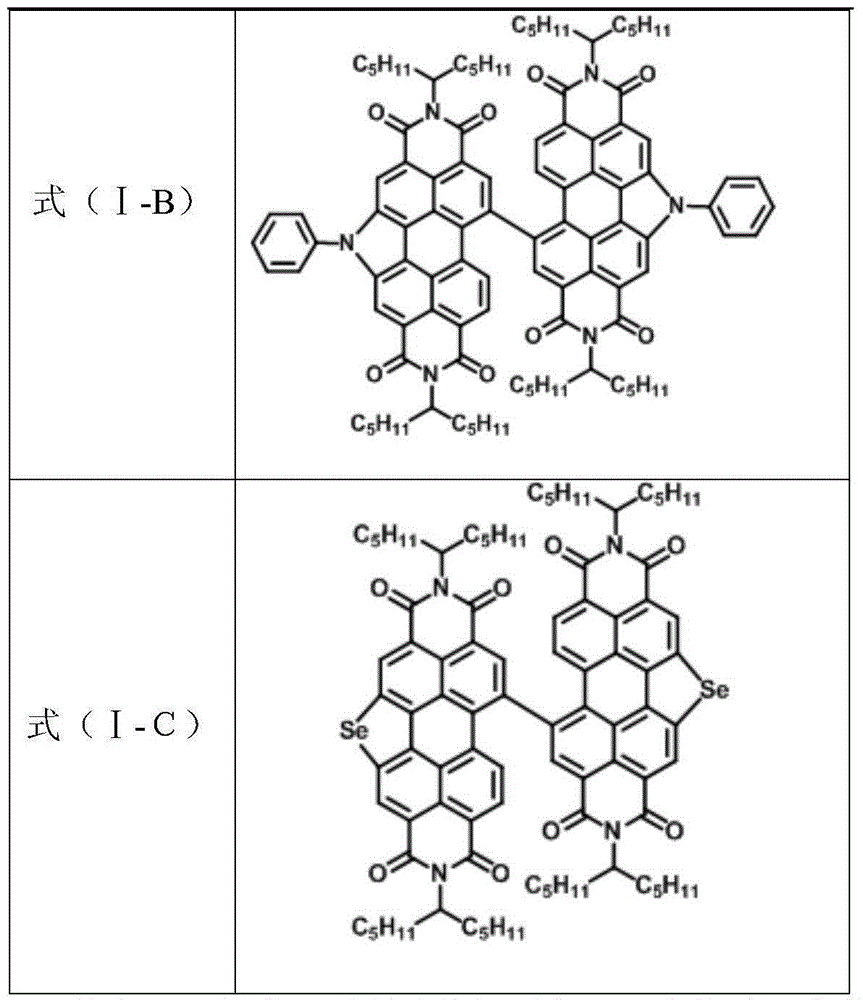
[0065] (3) Preparation of formula (I-B) compound
[0066] Add formula (IV-A) compound (250mg, 0.16mmol), palladium acetate (80mg, 0.36mmol), three (cyclohexyl) phosphorus (70mg, 0.25mmol) and potassium tert-butoxide (200mg, 1.79mmol) in 50ml two-necked bottle mmol), then add aniline (110mg, 1.18mmol) and toluene (10ml), under argon protection, reflux for 5h, cool to room temperature, remove the solvent under reduced pressure, wash the crude product with HCl, extract with dichloromethane, and separate the organic phase , washed with brine, dried over countless sodium sulfates, purified on a silica gel column, and dichloromethane / petroleum ether was used as a developing solvent to obtain the compound (153 mg) of formula (I-B) as a red solid 6 with a yield of 60%.
[006...
Embodiment 3
[0071] (1) Preparation of intermediate compound formula (Ⅲ-A): the method is the same as in Example 1;
[0072] (2) Preparation of intermediate compound formula (IV-A): the method is the same as in Example 1;
[0073] (3) Preparation of formula (I-C) compound:
[0074] Sodium selenide (99mg, 0.79mmol) was added to the DMF solution of the compound of formula (IV-A) (400mg, 0.26mmol), reacted at 120°C for 12h under the protection of argon, cooled to room temperature, poured into water, pumped The precipitate was collected by filtration, washed with water, dried, and purified on a silica gel column using dichloromethane / petroleum ether as a developing solvent to obtain compound (121 mg) of formula (I-C) as a red solid 7 with a yield of 30%.
[0075]
[0076] Characterization data of the compound of formula (I-C): 1 HNMR (400MHz, CDCl 3 )δ=9.62(d,2H),9.46(d,2H),8.73(d,2H),8.28(d,2H),8.16(t,2H),5.22(m,4H),2.29(m,8H ),1.88(m,8H),1.27(d,48H),0.81(m,24H); 13 CNMR (100MHz, CDCl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com