Neutral cellulase mutant and application thereof
A technology of cellulase and mutants, applied in application, fiber treatment, biochemical fiber treatment, etc., can solve the problems of few types of neutral cellulase, low catalytic efficiency, short product storage period, etc., and achieve fabric strength loss Small, good effect, stable batch difference
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Embodiment 1: Obtaining of cellulase mutants
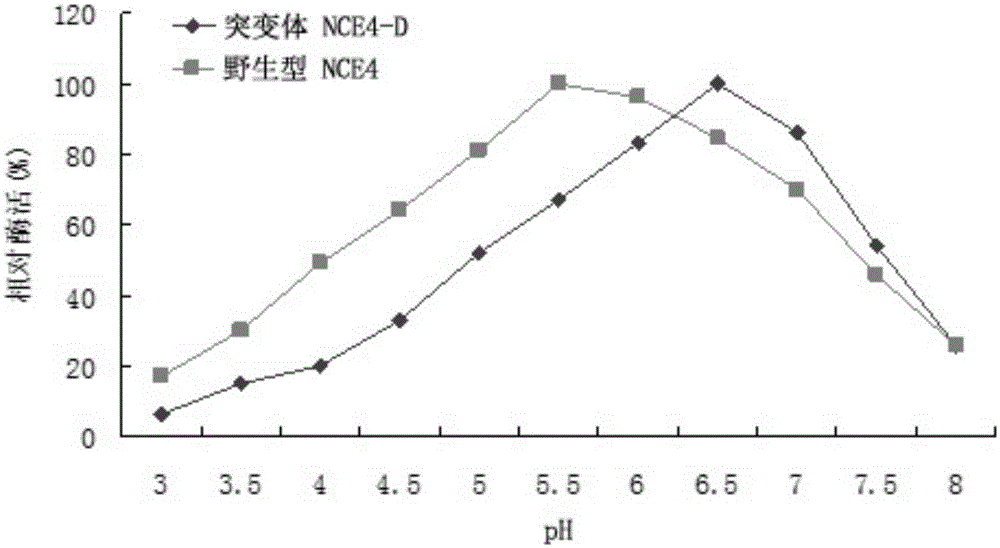
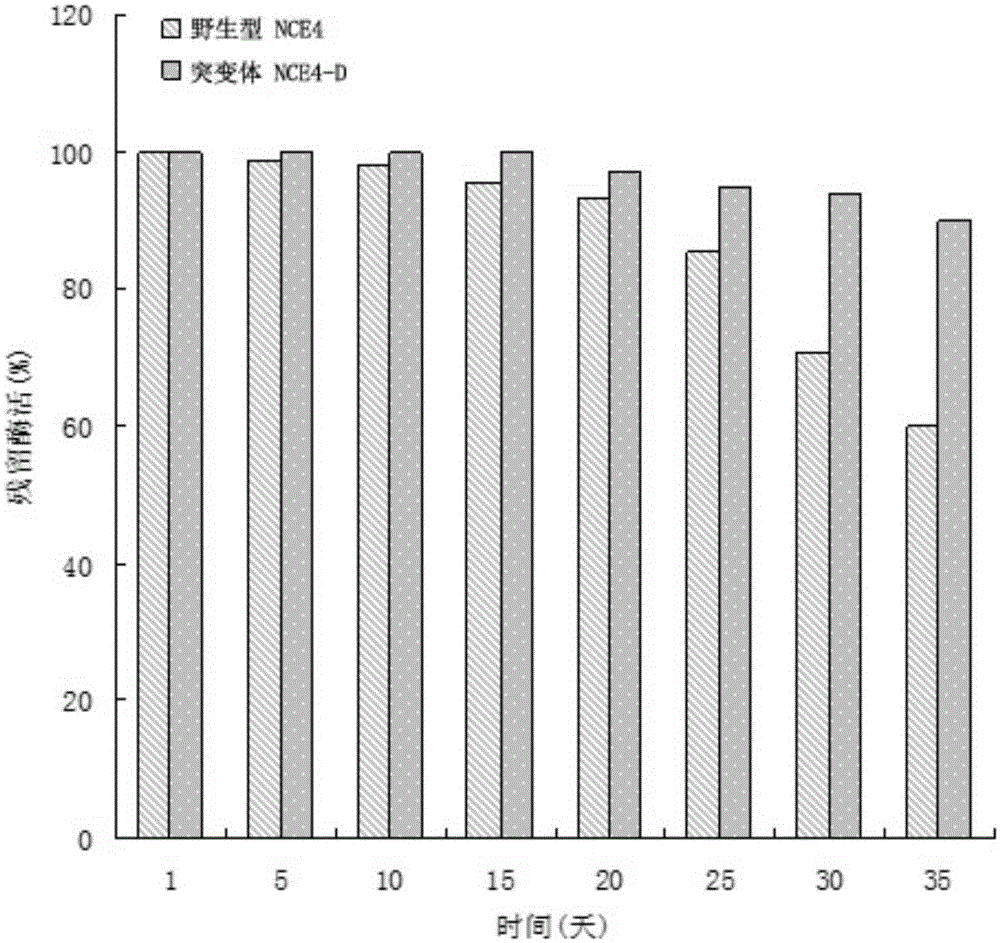
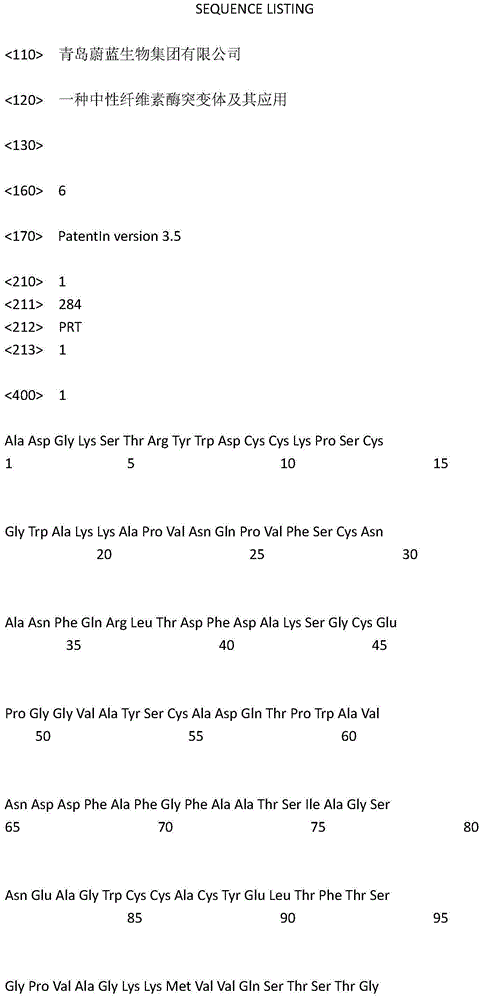
[0021] In order to improve the tolerance and stability of wild-type cellulase NCE4 (the amino acid sequence is SEQIDNO: 1, and its encoding nucleotide sequence is SEQIDNO: 2) to temperature, the applicant has carried out a large number of amino acid sites of the enzyme Point mutation screening, and extensive substitution screening of the zymogen's CBD-binding domain sequence. During the screening process, the applicant found that: some mutations have no effect on the heat resistance of cellulase, while some mutations can even lead to worse heat resistance, and some mutations can significantly improve the heat resistance of cellulase , but the enzyme activity level is very low, not suitable for industrial production. Finally, the applicant screened a combination of mutation sites and CBD binding domain sequences that can significantly improve the heat resistance and thermostability of cellulase without affecting its enzyme ...
Embodiment 2
[0026] Expression of embodiment 2 cellulase mutants
[0027] 2.1 Construction of expression vector
[0028] The synthesized cellulase mutant gene sequence and pTG vector were digested with restriction endonucleases KpnI and XbaI (Fermentas), respectively, and the digested products were purified using a gel purification kit, and T4DNA ligase (Fermentas) was used to The above two digested products were ligated and transformed into Escherichia coli Trans5α (Transgen), selected with ampicillin, and the clones were sequenced (Invitrogen). After the sequencing is correct, the recombinant vector pTG-NCE4-D containing the cellulase mutant gene is obtained.
[0029] The recombinant vector pTG-NCE4 containing the wild-type cellulase NCE4 gene was constructed by the same method as above.
[0030] 2.2 Construction and screening of recombinant strains
[0031] (1) Protoplast preparation
[0032] Inoculate Trichoderma reesei (Trichodermareesei) SCHD4 mycelia on a PDA plate, and culture ...
Embodiment 3
[0043] Embodiment 3 fermentation verification and enzyme activity assay
[0044] Trichoderma reesei NCE4-D (TrichodermareeseiNCE4-D) obtained by the above-mentioned construction and Trichodermareesei NCE4 (TrichodermareeseiNCE4) were respectively inoculated in MM fermentation medium (1.5% glucose, 1.7% lactose, 2.5% corn steep liquor , 0.44% (NH 4 ) 2 SO 4 , 0.09% MgSO 4 , 2% KH 2 PO 4 , 0.04% CaCl 2 , 0.018% Tween-80, 0.018% trace elements, 0.018% polypropylene glycol-2000), cultivated at 28°C for 48 hours, then cultivated at 25°C for 48 hours, centrifuged to get the supernatant. The enzyme activities were measured respectively.
[0045] (1) Enzyme activity assay method
[0046] Under the conditions of 50°C and pH value of 4.8 (neutral is pH 6.0), the amount of enzyme needed to degrade and release 1 μmol of reducing sugar from a sodium hydroxymethylcellulose solution with a concentration of 5 mg / ml per minute is one enzyme The unit of activity is U, and the reducing ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com