Preparation method of imipenem-cilastatin sodium sterile powder
The technology of cilastatin sodium and imipenem is applied in the field of preparing imipenem and cilastatin sodium aseptic powder, which can solve the problems of easy oxidative decomposition of active ingredients and the like, and achieves the advantages of being favorable for dispensing, having good fluidity, The effect of increasing the specific surface area
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
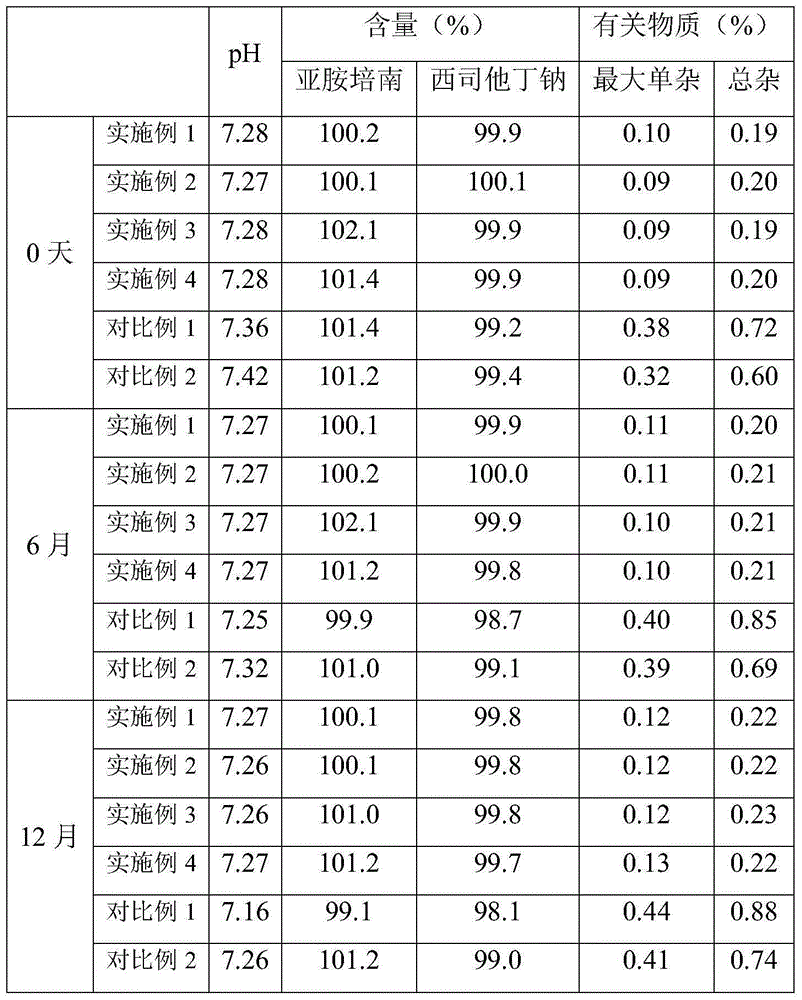
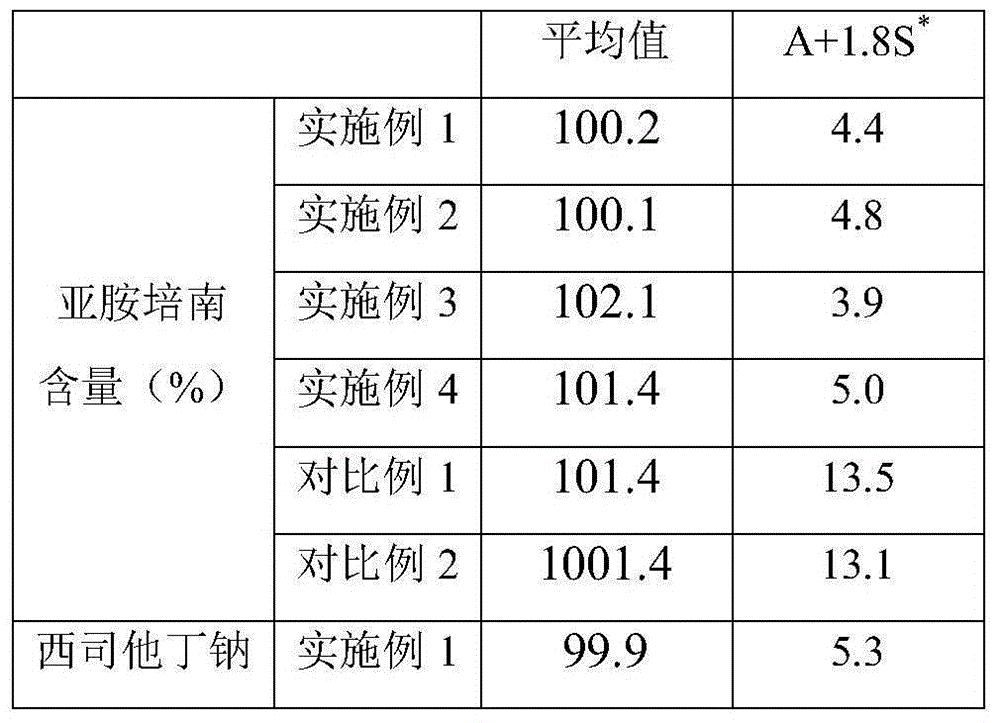
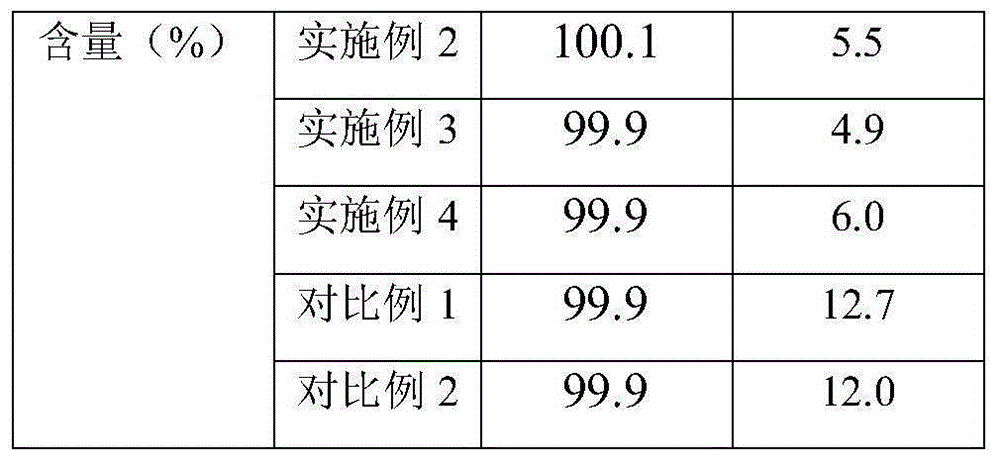
[0025] (1) Imipenem and cilastatin sodium are dissolved in the solvent in a weight ratio of 1:1, and the solution is sterilized and filtered with a 0.22um microporous membrane for subsequent use; the solvent used is water: acetone=1 :10. (2) Sterilize the cabinet of the spray dryer with pure steam hot-pressing at a sterilization temperature of 121° C. and a sterilization time of 20 minutes. After sterilization, dry it with nitrogen gas and cool it down for use. (3) Heat the feed liquid in (1) to 50°C, feed it into a spray dryer, and spray dry it under the condition of 50°C and nitrogen purging, and the pressure in the drying box is 350kPa. After the uniform granules were obtained, the moisture content, organic solvent residue, imipenem and cilastatin sodium in the granules were determined. (4) Pack the above-mentioned qualified particles into clean vials under the protection of nitrogen gas, fill the bottles with nitrogen gas, press the rubber stopper tightly, and roll the al...
Embodiment 2
[0027] (1) Dissolve imipenem and cilastatin sodium in a solvent in a weight ratio of 1:1, and filter the solution with a 0.22um microporous membrane for sterilization; for subsequent use; the solvent used is water: tert-butanol =1:30. (2) Sterilize the cabinet of the spray dryer with pure steam hot-pressing at a sterilization temperature of 121° C. and a sterilization time of 20 minutes. After sterilization, dry it with nitrogen gas and cool it down for use. (3) Heat the feed liquid in (1) to 55°C, feed it into a spray dryer, and spray dry it under the condition of 55°C and nitrogen purging, and the pressure in the drying box is 450kPa. After the uniform granules were obtained, the moisture content, organic solvent residue, imipenem and cilastatin sodium in the granules were determined. (4) Pack the above-mentioned qualified particles into clean vials under the protection of nitrogen gas, fill the bottles with nitrogen gas, press the rubber stopper tightly, and roll the alumi...
Embodiment 3
[0029] (1) Dissolve imipenem and cilastatin sodium in a solvent in a weight ratio of 1:1, and filter the solution with a 0.22um microporous membrane for sterilization; the solvent used is water: acetone: t Butanol=1:10:10. (2) Sterilize the cabinet of the spray dryer with pure steam hot-pressing at a sterilization temperature of 121° C. and a sterilization time of 20 minutes. After sterilization, dry it with nitrogen gas and cool it down for use. (3) Heat the feed liquid in (1) to 60°C, feed it into a spray dryer, and carry out spray drying under the condition of 60°C and nitrogen purging, and the pressure in the drying oven is 500kPa. After the uniform granules were obtained, the moisture content, organic solvent residue, imipenem and cilastatin sodium in the granules were determined. (4) Pack the above-mentioned qualified particles into clean vials under the protection of nitrogen gas, fill the bottles with nitrogen gas, press the rubber stopper tightly, and roll the alumin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com