A kind of nifedipine long-acting sustained-release pellets and preparation method thereof
A technology of sustained-release pellets and nifedipine, which is applied in the fields of cardiovascular system diseases, bulk delivery, drug combination, etc., and can solve the problem that the drug release rate cannot be effectively controlled, the solubility of nifedipine is small, and the release rate of sustained-release preparations Low-level problems, to achieve the effect of improving bioavailability, prolonging transit and absorption time, reducing aggregation and caking
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
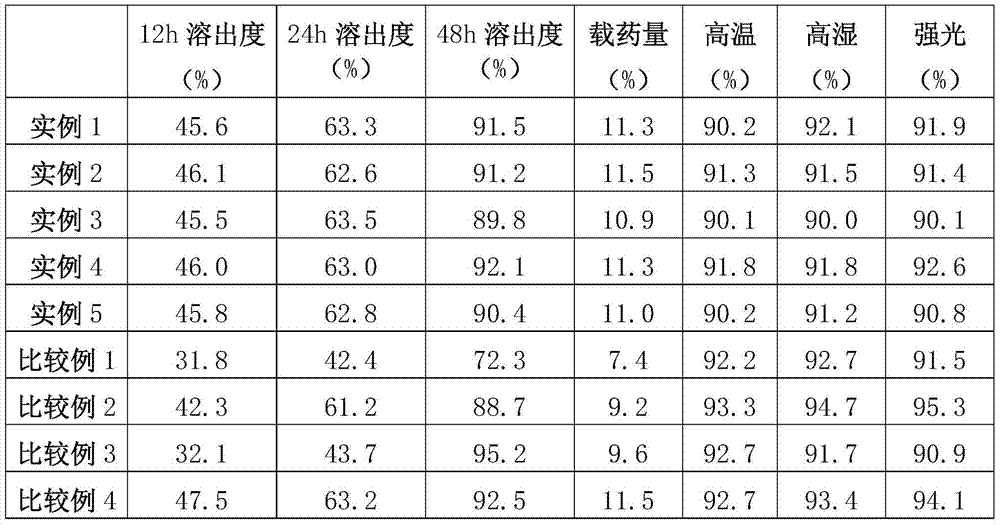
example 1
[0030] A preparation method of the nifedipine long-acting sustained-release pellets, comprising the steps of:
[0031] Step 1: Add 10 parts by weight of nifedipine into a mixed solvent of ethanol and dichloromethane and stir until dissolved to obtain a nifedipine solution, then take 19 parts by weight of polyethylene glycol 4000 and 10 parts by weight of sorbitol in a 95°C water bath After melting, add in the nifedipine solution, stir evenly, cool down and pulverize to obtain the nifedipine dispersion;
[0032] Step 2: After mixing the nifedipine dispersion with 49 parts by weight of welan gum, 5 parts by weight of stearic acid, 5 parts by weight of lactose, and 2 parts by weight of talcum powder, add 25 parts by weight of 15% aqueous ethanol to moisturize Wet to get soft material;
[0033] Step 3: extrude the soft materials through an extruder at a speed of 34r / min, and spheronize at a speed of 1100r / min in a spheronizer to prepare a spherical product, and dry at 45°C for 20...
example 2
[0035] A preparation method of the nifedipine long-acting sustained-release pellets, comprising the steps of:
[0036] Step 1: Add 12 parts by weight of nifedipine into a mixed solvent of ethanol and dichloromethane and stir until dissolved to obtain a nifedipine solution, then take 19 parts by weight of polyethylene glycol 4000 and 14 parts by weight of sorbitol in a water bath at 100°C After melting, add in the nifedipine solution, stir evenly, cool down and pulverize to obtain the nifedipine dispersion;
[0037] Step 2: After mixing the nifedipine dispersion with 30 parts by weight of welan gum, 10 parts by weight of stearic acid, 10 parts by weight of lactose, and 5 parts by weight of micropowdered silica gel, add 30 parts by weight of 20% ethanol aqueous solution to moisturize Wet to get soft material;
[0038] Step 3: Extrude the soft material through an extruder at a speed of 30r / min, and spheronize at a speed of 1080r / min to prepare a spherical product, and dry at 50°...
example 3
[0040] A preparation method of the nifedipine long-acting sustained-release pellets, comprising the steps of:
[0041] Step 1: Add 10 parts by weight of nifedipine into a mixed solvent of ethanol and dichloromethane and stir until dissolved to obtain a nifedipine solution, then take 18 parts by weight of polyethylene glycol 4000 and 12 parts by weight of sorbitol in a 97°C water bath After melting, add in the nifedipine solution, stir evenly, cool down and pulverize to obtain the nifedipine dispersion;
[0042] Step 2: After mixing the nifedipine dispersion with 42 parts by weight of welan gum, 7 parts by weight of stearic acid, 8 parts by weight of lactose and 3 parts by weight of sodium lauryl sulfate, add 27 parts by weight of 17% Wetting the ethanol aqueous solution to obtain soft materials;
[0043] Step 3: Extrude the soft material through an extruder at a speed of 32r / min, and spheronize at a speed of 1050r / min to prepare a spherical product, and dry it at 47°C for 25 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com