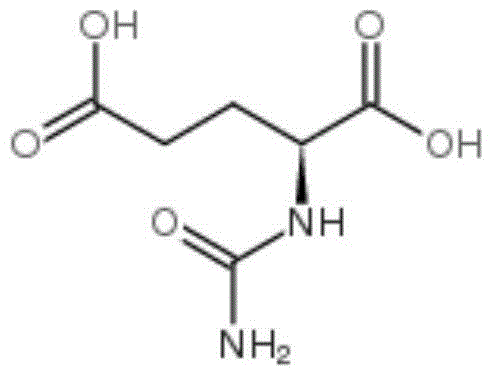
Carglumic acid solid composition and preparation method thereof
A technology of solid composition and carboglutamic acid, which is applied in the direction of drug combination, pill delivery, anti-toxic agent, etc., can solve the poor moisture and heat stability of the raw material drug, easily cause the degradation of the main drug and the production of related substances, and the production process requirements of the preparation Higher problems, to achieve the effect of low environmental requirements, stable and reliable quality, and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
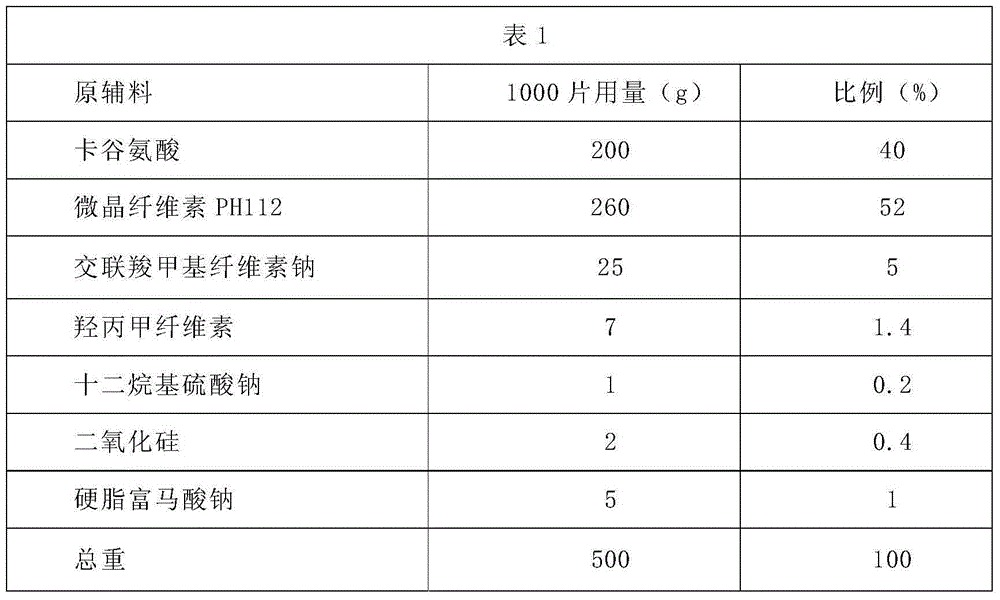
[0024] Carglutamic acid tablets prescription composition and direct compression method preparation process:
[0025]
[0026] Preparation:
[0027] 1. Pass the carboglutamic acid through a 40-mesh sieve, and set aside;
[0028] 2. Dry the above-mentioned microcrystalline cellulose, croscarmellose sodium, and hypromellose at 60°C for about 2 hours, and pass through a 60-mesh sieve;
[0029] 3. Put carboglutamic acid, dried microcrystalline cellulose, croscarmellose sodium, and hypromellose in a mixer and mix for several minutes until uniform;
[0030] 4. Pass sodium lauryl sulfate and silicon dioxide through a 60-mesh sieve, add to the mixed material in step 3, and continue to mix evenly;
[0031] 5. Sodium stearyl fumarate is passed through a 80-mesh sieve, and added to the mixed material in step 4. After mixing evenly, measure the moisture content; when the moisture content is less than 2%, it is qualified;
[0032] 6. Press the tablet after passing the moisture test, a...
Embodiment 2
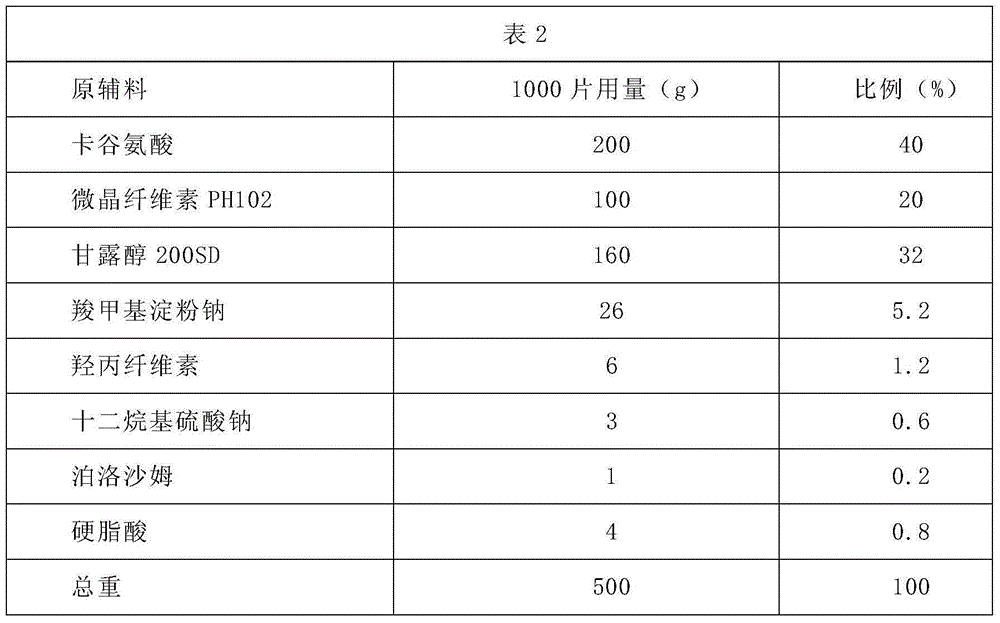
[0035] Carglutamic acid tablets prescription composition and direct compression method preparation process:
[0036]
[0037] Preparation:
[0038] 1. Pass the carboglutamic acid through a 40-mesh sieve, and set aside;
[0039] 2. Dry the above-mentioned microcrystalline cellulose, mannitol, sodium carboxymethyl starch, and hydroxypropyl cellulose at 60°C for about 2 hours, and pass through a 60-mesh sieve;
[0040] 3. Put carboglutamic acid and the above-mentioned dried microcrystalline cellulose, mannitol, sodium carboxymethyl starch, and hydroxypropyl cellulose in a mixer and mix for several minutes until uniform;
[0041] 4. Pass sodium lauryl sulfate and poloxamer through a 60-mesh sieve, add to the mixture in step 3, and continue to mix evenly;
[0042] 5. Pass stearic acid through a 80-mesh sieve, add it to the mixed material in step 4, and measure the moisture content after mixing evenly; it is qualified when the moisture content is less than 2%;
[0043] 6. Pres...
Embodiment 3
[0046] Carglutamic acid tablets prescription composition and direct compression method preparation process:
[0047]
[0048]
[0049] Preparation:
[0050] 1. Pass the carboglutamic acid through a 40-mesh sieve, and set aside;
[0051] 2. Dry the above-mentioned microcrystalline cellulose, mannitol, cross-linked polyvinylpyrrolidone, and hypromellose at 60°C for about 2 hours, and pass through a 60-mesh sieve;
[0052] 3. Put carboglutamic acid and the above-mentioned dried microcrystalline cellulose, mannitol, cross-linked polyvinylpyrrolidone, and hypromellose in a mixer and mix for several minutes until uniform;
[0053] 4. Pass sodium lauryl sulfate and silicon dioxide through a 60-mesh sieve, add to the mixed material in step 3, and continue to mix evenly;
[0054] 5. Pass the talcum powder through an 80-mesh sieve, add it to the mixed material in step 4, and measure the moisture content after mixing evenly; it is qualified when the moisture content is less than ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com