Phloroglucinol derivatives and isomers separation and preparation method and application
A technology of phloroglucinol and phloroglucinol, which is applied in the field of chemistry and medicine, and achieves the effects of high purity, convenient operation and good medicinal activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] The separation and preparation of embodiment 1 phloroglucinol derivatives
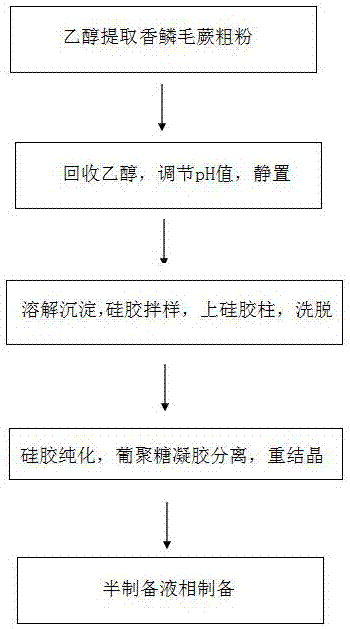
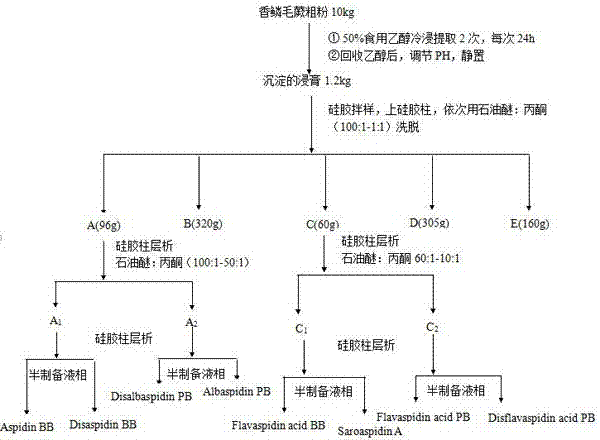
[0056] The schematic diagram of the separation and preparation is shown in the appendix figure 1 And attached figure 2 shown.
[0057] S1. Extraction of the total phloroglucinol derivatives of Trichomonas chinensis: extract 10 kg dry coarse powder of Trichomonas 10 times by cold immersion in 50% edible ethanol twice, each time for 24 hours, concentrate and recover under reduced pressure Ethanol to alcohol-free sample aqueous solution;
[0058] S2. Adjust the pH to 1.5-4.5 with hydrochloric acid, let stand for 12-18 hours, and centrifuge to obtain 1.2 kg of precipitated extract.
[0059] S3. After dissolving the precipitated extract sample obtained in S2, mix the sample with silica gel, perform preliminary separation on the silica gel column, and successively use different proportions of petroleum ether: acetone to elute (100:1-1:1) to obtain the corresponding components Fr.A~E.
[0060] Th...
Embodiment 2
[0064] The separation and preparation of embodiment 2 phloroglucinol derivatives
[0065] The schematic diagram of the separation and preparation is shown in the appendix figure 1 And attached figure 2 shown.
[0066] S1. Extraction of the total phloroglucinol derivatives of Trichomonas chinensis: extract 10 kg dry coarse powder of Trichomonas 10 times by cold immersion in 50% edible ethanol twice, each time for 24 hours, concentrate and recover under reduced pressure Ethanol to alcohol-free sample aqueous solution;
[0067] S2. Adjust the pH to 1.5-4.5 with hydrochloric acid, let stand for 12-18 hours, and centrifuge to obtain 1.2 kg of precipitated extract.
[0068] S3. After dissolving the precipitated extract sample obtained in S2, mix the sample with silica gel, perform preliminary separation on the silica gel column, and successively use different proportions of petroleum ether: acetone to elute (100:1-1:1) to obtain the corresponding components Fr. A-E.
[0069] S...
Embodiment 3
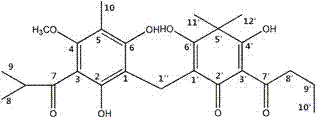
[0071] Example 3 In vitro anti-superficial fungus effect of Phloroglucinol 4 pairs of isomer compounds
[0072] (1) Trichophyton rubrum (CMCC(f)T1b) and Microsporum gypsumoidus (CMCC(F)M2C), provided by the Institute of Dermatology, Chinese Academy of Medical Sciences. The microdilution method of dermatophytes was carried out according to the M38-A2 protocol formulated by CLSI in the United States, which is briefly described as follows. Gently scrape the colonies on the surface of the SDA medium with an inoculation loop and grind the mycelium with a sterile grinder, suspend the dermatophyte in sterile saline, adjust the turbidity to 0.5 McFarland turbidity, and count the number of spores with a hemocytometer and short mycelia. Make the bacterial concentration 1×10 3 CFU / ml to 3×10 3 CFU / ml. That is, the 0.5 McFarland turbidity bacteria solution was diluted 1000 times with RPMI-1640 liquid medium, and counted by the hemocytometer to obtain the inoculum solution. Use RPMI-1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com