Fusion protein for detecting anti-ETEC (enterotoxigenic escherichia coil) antibody of pigs, as well as preparation method and application thereof
A technology of fusion protein and Escherichia coli, which is applied in the direction of chemical instruments and methods, and the use of vectors to introduce foreign genetic material, bacteria, etc., can solve the problems of cumbersome purification methods of adhesin, easy cross-reaction, low purity and yield, and achieve immunity Good reactivity, low cost, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
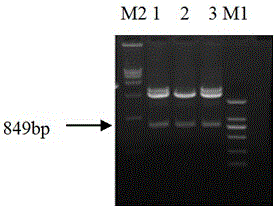
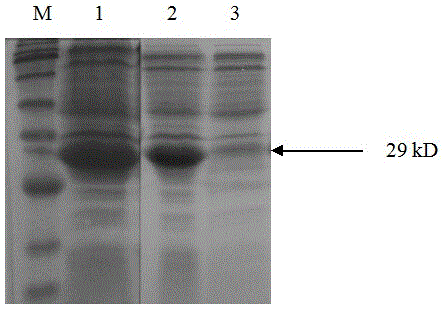
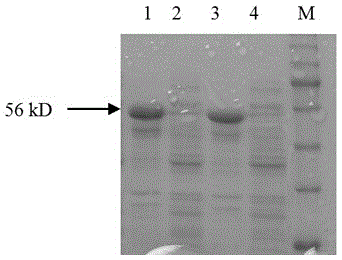
[0022] Example 1 Preparation of fusion protein for detection of anti-ETEC antibodies
[0023] 1. Fusion gene designed to detect anti-ETEC antibody
[0024] The nucleotide and amino acid sequences of ETECF4(K88) pili and ETECF5(K99) pili were analyzed by the biological software DNAStar. After analysis using the codon optimization software CodonAdaptationTool (JCAT), replace the rare codons of E. coli in the nucleotide sequences of F4 and F5 pili with preferred codons, and add a linker sequence between the gene fragments of F4 and F5 pili, The fusion gene was designed, and the sequence is shown in SEQ ID NO:2. Add an EcoRI restriction site at the 5' end of the fusion gene, add a SalI restriction site at the 3' end, and send it to Nanjing GenScript Biotechnology Company for synthesis.
[0025] The fusion genes encode fusion proteins F4-F5 for detection of anti-ETEC antibodies. The amino acid sequence of the fusion protein F4-F5 is shown in SEQ ID NO:1.
[0026] 2. Constructio...
Embodiment 2
[0039] Embodiment 2 Detects the composition and method of use of the anti-ETEC antibody kit
[0040] 1. The composition of the kit
[0041] 1. Washing solution: prepare pH 7.4, 0.01M phosphate buffer solution, and add Tween-20 with a final concentration of 0.05%. Specific preparation method: weigh KH 2 PO 4 0.2g, Na 2 HPO 4 12H 2 Dissolve O2.9g and NaCl8g in water, then dilute to 1000mL, add 0.5mL Tween-20.
[0042] 2. Diluent: pH7.4, 0.01M phosphate buffer, the preparation method is the same as above.
[0043] 3. Stop solution: 2M sulfuric acid solution, dilute 111.2mL of concentrated sulfuric acid with water and dilute to 1000mL.
[0044] 4. Substrate chromogenic solution: soluble TMB one-component substrate chromogenic solution (purchased from Nanjing Zhuyan Biotechnology Co., Ltd.).
[0045] TMB is an abbreviation for 3,3’,5,5’-tetramethylbenzidine.
[0046] 5. ETEC-positive serum standard product: from multiple ETEC-immunized piglet sera, select a micro-agglutina...
Embodiment 3
[0080] Embodiment 3 specificity experiment
[0081] Investigate the specificity of the kit in Example 2. Adopt kit in embodiment 2 to know PEDV (porcine epidemic diarrhea virus), PRRSV (porcine reproductive and respiratory syndrome virus), PRV (pseudorabies virus), CSFV (swine fever virus), PCV2 with indirect ELISA method (Porcine circovirus type 2), APP (Actinobacillus pleuropneumoniae) and SS (Streptococcus suis) positive sera were tested. Three parallel replicates were performed for each serum. The OD value of each well was measured with a microplate reader at a wavelength of 450 nm to determine the specificity of the indirect ELISA method. The positive sera of PEDV, PRRSV, PRV, CSFV, and PCV2 were purchased from IDEXX Company, and the positive sera of APP and SS were purchased from Wuhan Keqian Biotechnology Co., Ltd. The results showed that the S / P of PEDV, PRRSV, PRV, CSFV, FMDV, PCV2, APP, and SS positive sera were all less than 0.23, which was judged as negative.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com