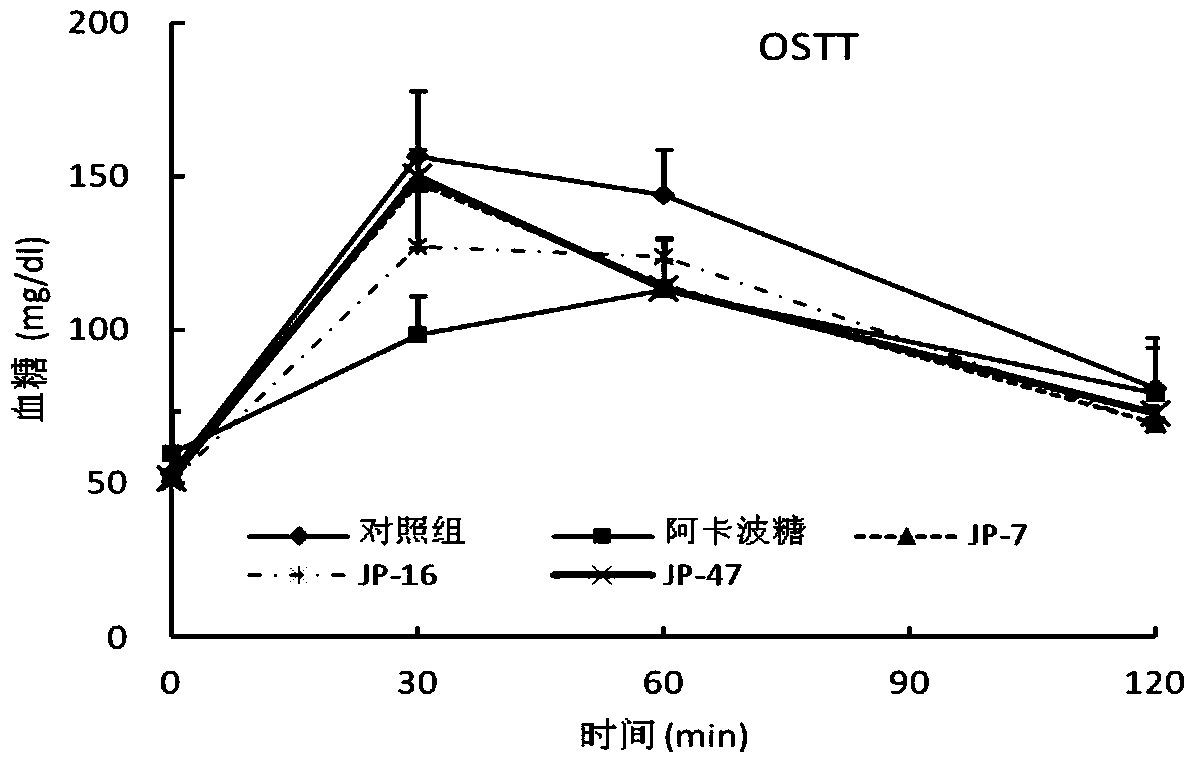
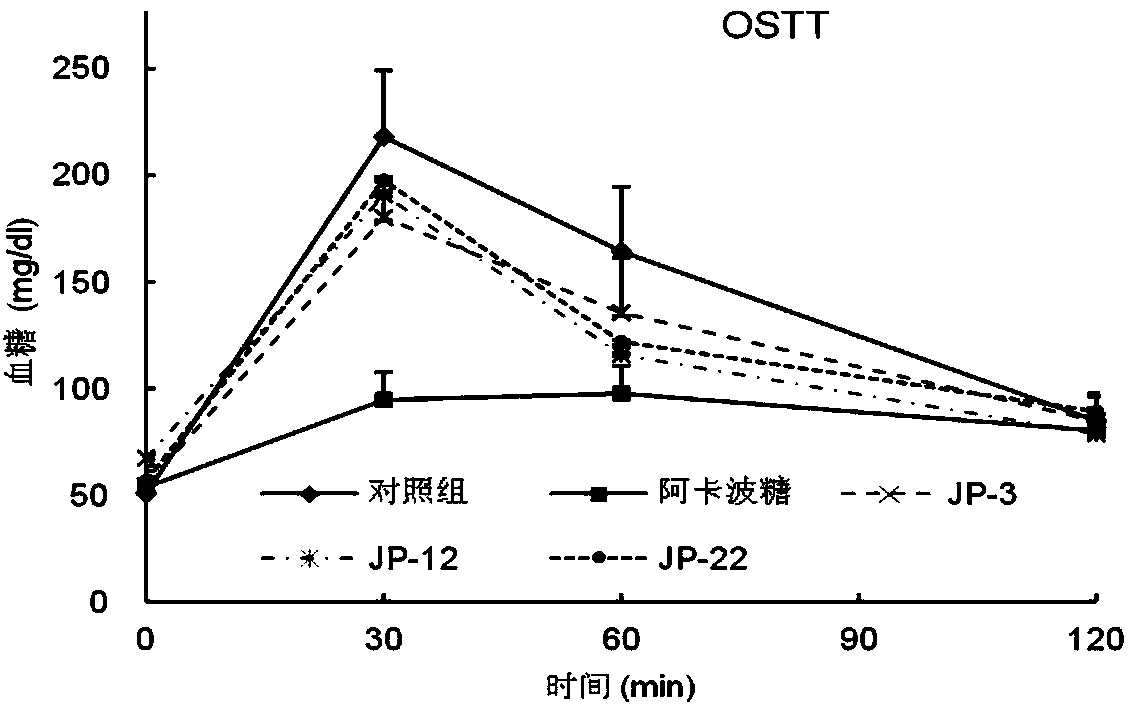
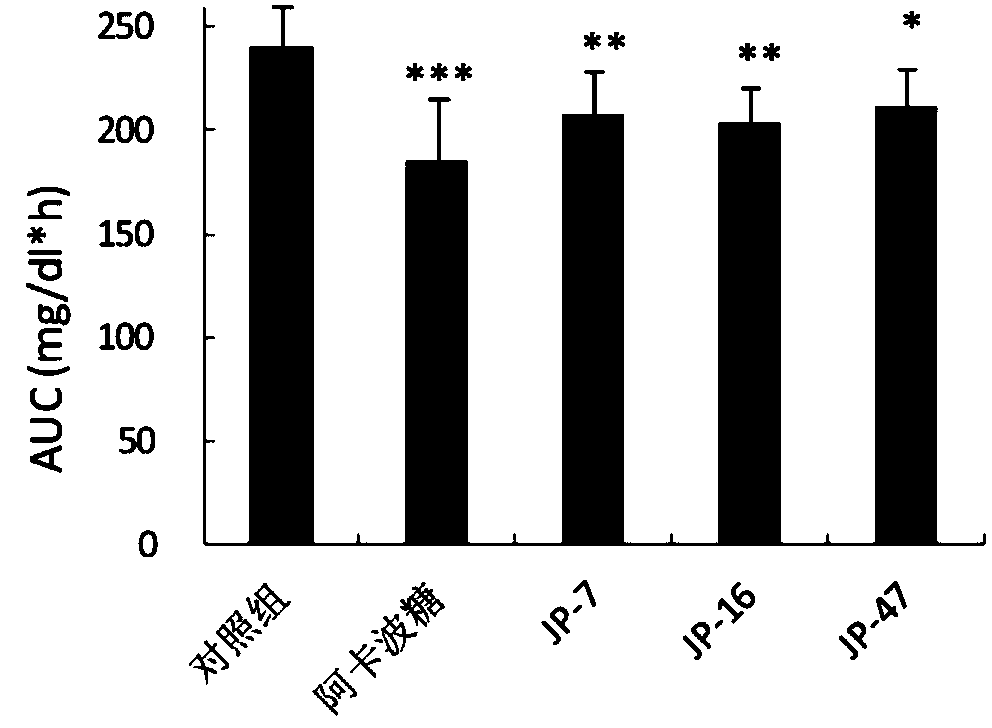
Flavane compound, preparation method thereof and hypoglycemic activity
A compound and flavan technology, applied in the field of medicine, can solve the problems of immature research, complex structure, and difficult purification of monomer compounds.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0103] The extraction of preparation example 1 flavan polymer part
[0104] The extracting of this part refers to the Chinese patent before, and the application number is the extraction method of the effective part of Rhodiola rosea of 201010587820.7, collect each 20kg of Rhodiola rosea and Rhodiola angustifolia, concentrate after 3 times of 80% ethanol extraction, obtain extract, and then Add distilled water with a mass ratio of 10 times the amount to the extract and let it stand for 96 hours at about 10°C. After the supernatant is concentrated, the macroporous resin is separated through four gradients of water, 5% ethanol, 50% ethanol, and 95% ethanol in sequence. The 50% water-ethanol fraction was concentrated and freeze-dried to obtain two fractions rich in different types of flavan polymers.
preparation example 2
[0105] The preparation of preparation example 2 flavan derivatives
[0106] Take a certain amount of flavan polymer parts of Dahua or Rhodiola angustifolia, dissolve it in anhydrous methanol according to the mass ratio of 1:30, and add the corresponding thio reagent according to the mass ratio of 1:0.5, and the mass ratio of 1 Add 48% HBr aqueous solution at a ratio of 0.5, react at 60°C for 4h, suspend the reaction solution with distilled water with a mass ratio of 5:1 to the reaction solution, extract 3 times with ethyl acetate, combine the organic layers, and organic After the layer was dried and concentrated under reduced pressure, the 200-300 mesh silica gel column separation and LH-20 column chromatography separation were carried out in sequence, and the methanol aqueous solution with a mass fraction of 30-70% was used to efficiently prepare the liquid phase to obtain the 4-position thio-substituted flavan derivative At present, a total of 51 new compounds are obtained, ...
Embodiment 14
[0116] Example 14- Preparation of (S)-(2,4-dichlorobenzylthio)-epicatechin gallate (JP-1)
[0117] The preparation method is the same as that of Preparation Example 2, the flavan polymer raw material used is 50% part of Rhodiola rosea, and the 2,4-dichlorobenzylthiol used as the thio reagent is used to efficiently prepare 70% of the liquid phase (MeOH / H 2 (2 volume ratio) to obtain compound JP-1, light red powder, yield 32.1%. The NMR data of the compound are as follows:
[0118] (c0.1MeOH); HR-ESI-MS (m / z633.0386[M+H] + ,calcd,633.0383); 1 HNMR (500MHz, DMSO-d 6 )δ: 7.65 (1H, s, 3″’-H), 7.62 (1H, dd, J=8.0, 1.5, 5″’-H), 7.41 (1H, d, J=8.0, 6″’-H ),6.87(1H,d,J=1.5,2′-H),6.82(2H,s,2″,6″-H),6.72(1H,dd,J=8.0,1.5,6′-H) ,6.68(1H,d,J=8.0,5′-H),5.91(1H,d,J=2.0,8-H),5.83(1H,d,J=2.0,6-H),5.44(1H ,s,3-H),5.27(1H,s,2-H),4.12(1H,s,4-H),4.10(2H,s,S-C-H); 13 CNMR (125MHz, DMSO-d 6 )δ: 165.2(O-C-O), 158.1(C-5), 157.2(C-7), 155.4(C-9), 145.4(C-3″,5″), 144.9(C-3′,4′) ,138.9(C-4″),13...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com