Truncated rotavirus vp8 protein and use thereof
A rotavirus and protein technology, applied in the direction of viral peptides, antiviral agents, viral antigen components, etc., can solve problems such as inappropriate and efficient anti-rotavirus vaccines
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0177] Example 1: Construction of an expression vector encoding a truncated rotavirus VP8 protein
[0178] The rotavirus LLR strain was cultured with Rhesus monkey embryonic kidney cell line (MA-104). The medium used was DMEM supplemented with 2 μg / ml trypsin, 0.5 mg / ml ampicillin and 0.4 mg / ml streptomycin, 3.7 mg / ml sodium bicarbonate, 0.34 mg / ml L-glucose Aminoamide.
[0179] According to the manufacturer's instructions, the genomic RNA of rotavirus was extracted using the viral DNA / RNA extraction kit produced by Beijing Kingmag Biotechnology Co., Ltd., and the cDNA encoding the VP4 protein was obtained by reverse transcription. The obtained cDNA is used as a template, and the gene fragment encoding the truncated rotavirus VP8 protein is amplified by PCR reaction.
[0180] The primers used were as follows:
[0181] Upstream primers:
[0182] 5'- GGATCCCATATG ATACAGTTAATTGGATCAGAAAA-3' (SEQ ID NO: 17)
[0183] 5'- GGATCCCATATG GGATCAGAAAAAACGCAG-3' (SEQ ID NO: 18) ...
Embodiment 2
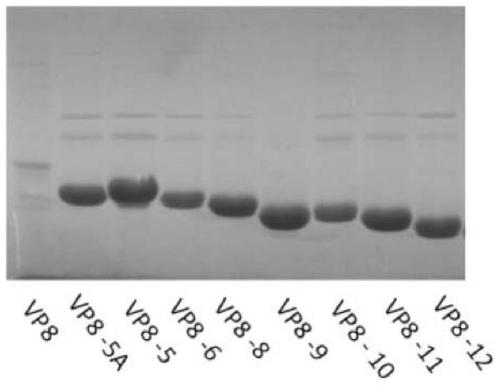
[0207] Example 2: Expression of truncated rotavirus VP8 protein
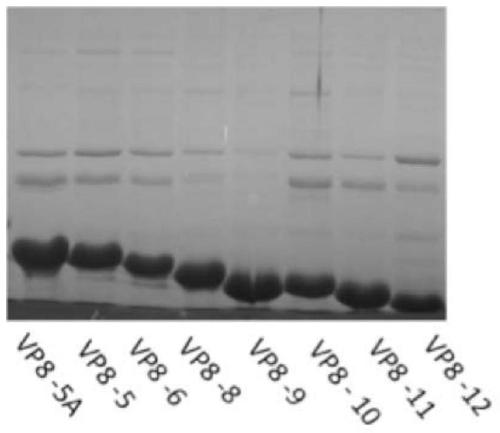
[0208] Take out the recombinant plasmids pTO-T7-VP8-5A, pTO-T7-VP8-5, pTO-T7-VP8-6, pTO-T7-VP8-8, pTO-T7-VP8 prepared in Example 1 from -70°C -9, pTO-T7-VP8-10, pTO-T7-VP8-11 or pTO-T7-VP8-12 Escherichia coli bacterial liquid, inoculate it into 50ml LB liquid medium containing kanamycin, in Cultivate at 180rpm at 37°C for about 4 hours; then transfer to 10 bottles of 500ml LB medium containing kanamycin (500ul bacteria solution in each bottle). When the absorbance value of the culture at 600 nm reached 0.5, IPTG was added to a final concentration of 1 mM, and culture was continued for 6 hours at 180 rpm at 25°C.
[0209] In addition, recombinant proteins PTO-T7-VP8, PTO-T7-ΔVP8* were also expressed using Escherichia coli by a method similar to the above.
Embodiment 3
[0210] Example 3: Purification and Characterization of Truncated Rotavirus VP8 Protein
[0211] Under the condition of Tris-HCl 8.0 buffer solution, at 4°C, use a sonicator (Thermo) according to the condition of 4min / 1g wet bacteria, destroy the cell wall of E.coli cells, collect the soluble fraction, and use the following scheme to analyze The recombinant protein expressed by E.coli cells was purified.
[0212] Instrument system: AKTAexplorer 100 preparative liquid chromatography system produced by GE Healthcare (formerly Amershan Pharmacia).
[0213] Chromatography medium: Q-sepharose-HP (GE Healthcare).
[0214] Column volume: 5.5cm*20cm.
[0215] Buffer: 50mM Tris-HCl pH 8.0
[0216] 50mM Tris-HCl pH 8.0, 2M NaCl
[0217] Flow rate: 25mL / min.
[0218] Detector wavelength: 280nm.
[0219] The sample was the soluble fraction of E. coli lysate prepared previously containing recombinantly expressed VP8-5A, VP8-5, VP8-6, VP8-8, VP8-9, VP8-10, VP8-11 or VP8-12.
[0220] T...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com