Novel Chiral Multidentate Ligands, Coordination-Formed Metal-Organic Coordination Polymer Catalysts and Their Applications
A coordination polymer, metal-organic technology, applied in the direction of organic compound/hydride/coordination complex catalyst, physical/chemical process catalyst, organic chemistry, etc., can solve the problem of catalytic activity and selectivity reduction, catalyst loading uniformity Poor, easy deactivation of the catalyst, etc., to achieve high catalytic activity and selectivity, good stability, and high catalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
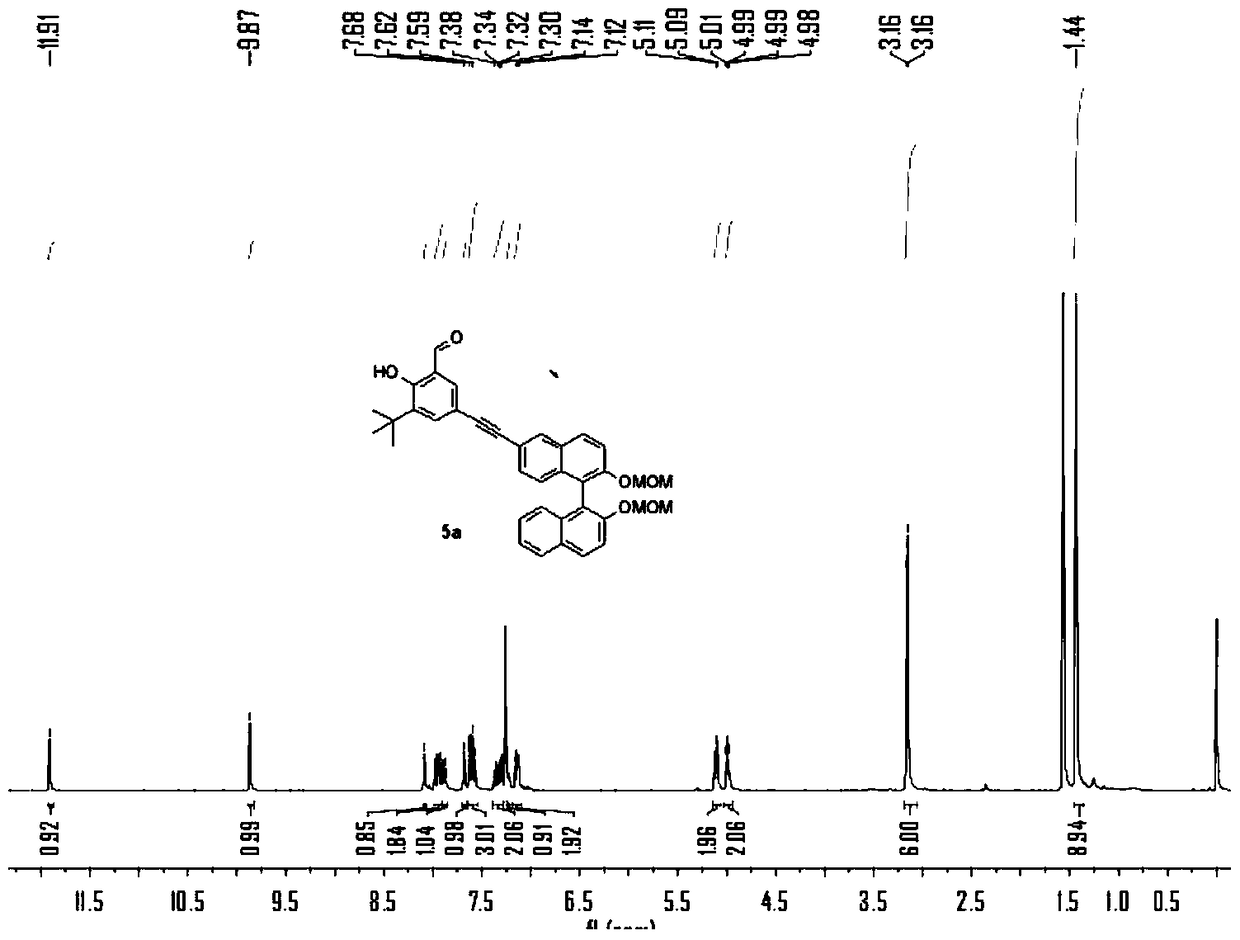
[0072] The preparation of embodiment 1 compound 3a
[0073]
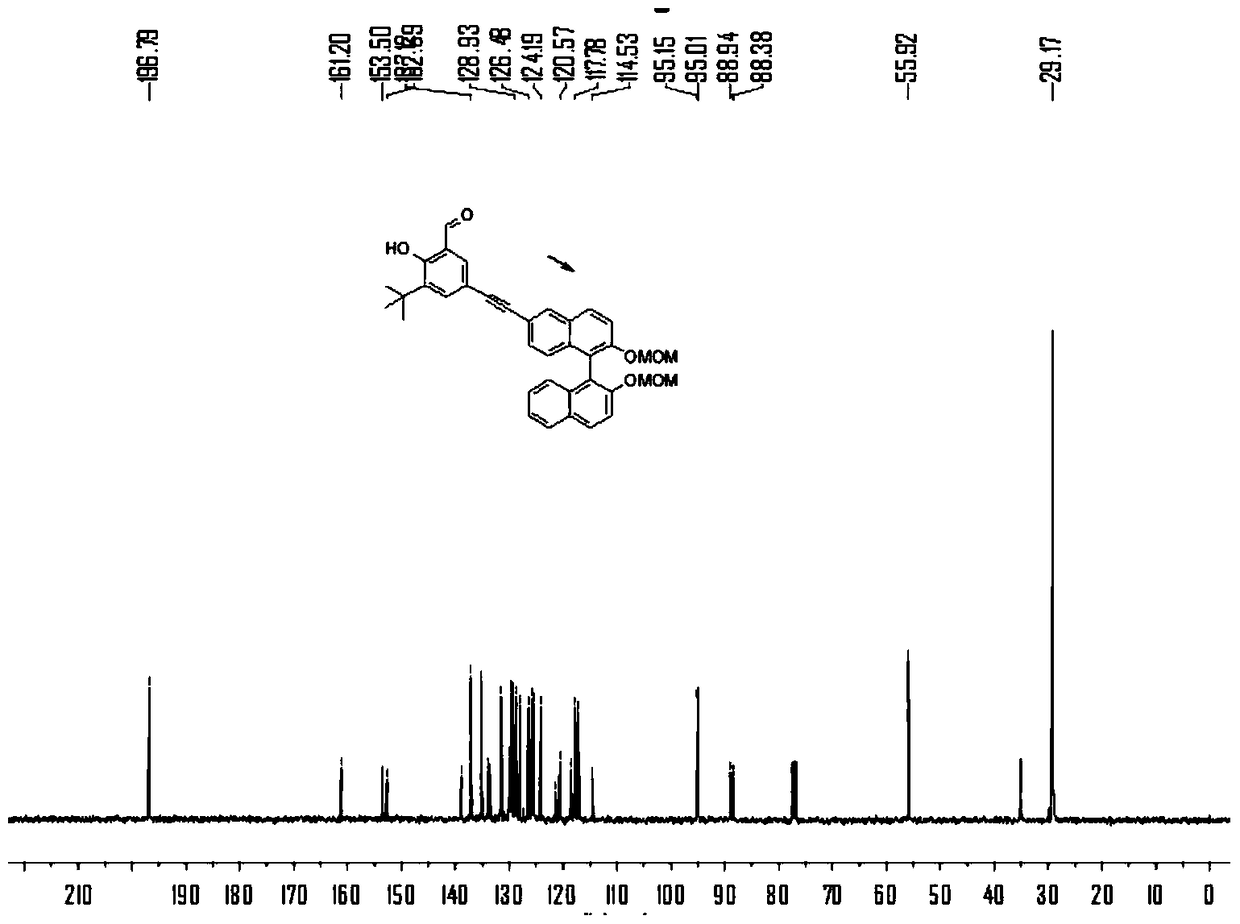
[0074] N 2 Under protection, add compound 1a (2.2g), compound 2 (4.5g), PdCl 2 (PPh 3 ) 2 (350mg), PPh 3 (130mg), triethylamine 10mL and tetrahydrofuran 30mL, stirred at room temperature for 1 hour. Then CuI (180mg) was added to the solution, heated to 80°C and stirred for 24h, TLC detected that compound 1a disappeared, and the reaction stopped. After purification by column chromatography, the product 3 was obtained as a yellow oil, 2.5 g, and the yield was 45%. 1 H NMR (400MHz, CDCl 3 ,298K)δ=11.92(s,1H),9.87(s,1H),8.09(d,J=1.4,1H),7.95(dd,J=15.2,9.0,2H),7.89(d,J=8.1 ,1H),7.68(d,J=1.9,1H),7.63(s,1H),7.63(d,J=1.3,1H),7.60(s,1H),7.58(s,0H),7.39–7.34 (m,1H),7.31(dd,J=8.8,1.6,1H),7.24(dd,J=6.8,1.3,1H),7.14(dd,J=8.3,6.2,2H),5.14–5.07(m ,2H),5.00(dd,J=6.8,4.4,2H),3.16(d,J=1.6,6H),1.44(s,9H). 13 C NMR (100MHz, CDCl 3 ,298K)δ=196.79,161.20,153.50,152.69,138.91,137.12,135.16,133.95,133.57,131.48,129.90,129.65,...
Embodiment 2
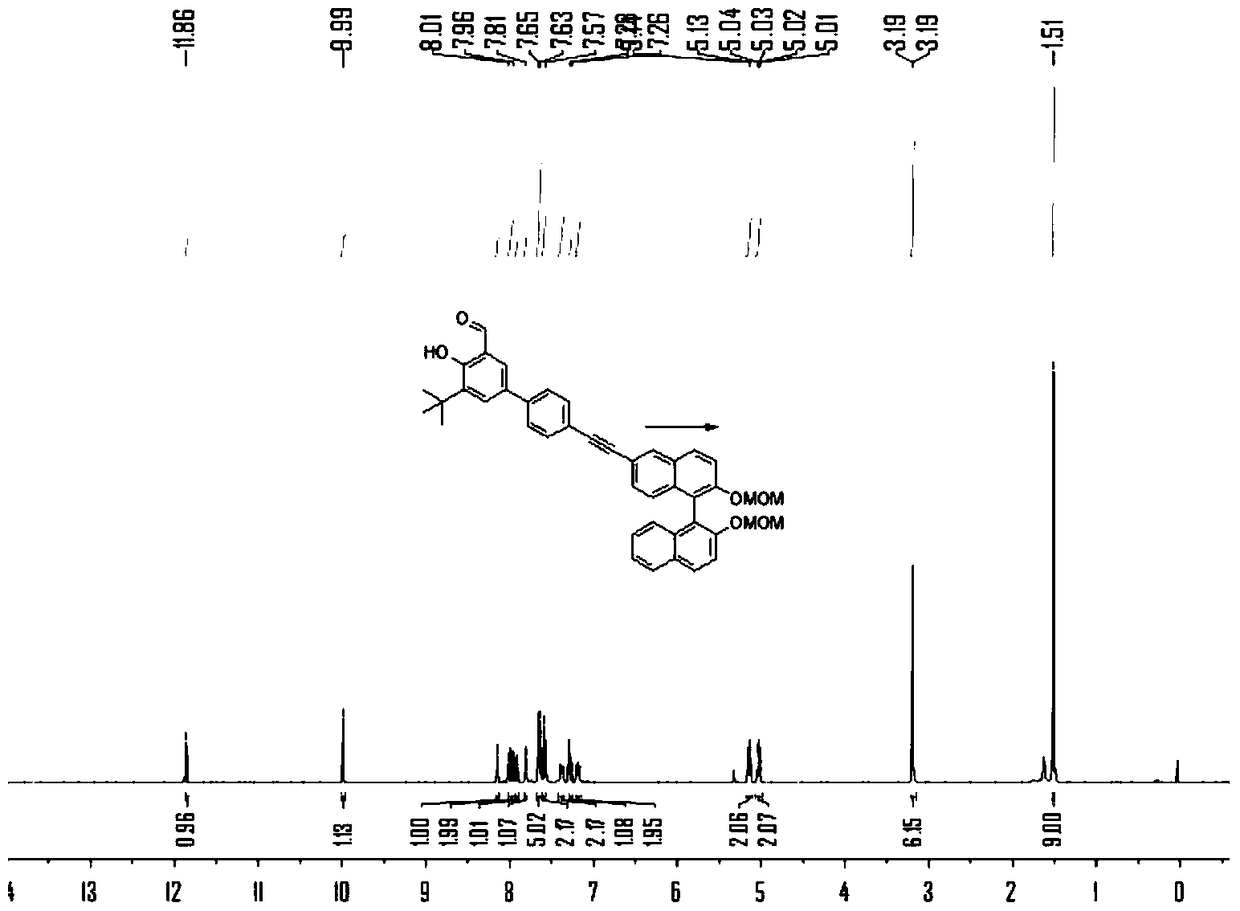
[0075] The preparation of embodiment 2 compound 3b
[0076]
[0077] N 2 Under protection, add compound 1b (2.8g), compound 2 (4.5g), PdCl 2 (PPh 3 ) 2 (350mg), PPh 3 (130 mg), 10 mL of triethylamine and 30 mL of tetrahydrofuran, stirred at room temperature for 1 hour. Then CuI (180 mg) was added to the solution, heated to 80° C. and stirred for 24 h, TLC detected that compound 1b disappeared, and the reaction stopped. After purification by column chromatography, the product 3b was obtained as a yellow oil, 5.2 g, and the yield was 88%. 1 H NMR (400MHz, CDCl 3 )δ=11.86(s,1H),9.99(s,1H),8.15(d,J=1.1,1H),7.99(dd,J=11.3,9.1,2H),7.92(d,J=8.1,1H ),7.81(d,J=2.2,1H),7.68–7.62(m,5H),7.60(d,J=7.8,2H),7.42–7.35(m,2H),7.27(dd,J=6.8, 1.2,1H),7.18(dd,J=8.6,4.1,2H),5.19–5.10(m,2H),5.03(dd,J=6.8,4.0,2H),3.19(s,3H),3.19(s ,3H),1.51(s,9H). 13 C NMR (101MHz, CDCl 3 )δ=197.25,160.90,153.53,152.70,139.72,138.98,133.96,133.62,132.89,132.21,131.61,130.04,129.91,129.64,129.39,129.01,12...
Embodiment 3
[0078] The preparation of embodiment 3 compound 4a:
[0079]
[0080] Compound 3a (574 mg), 15 mL of chloroform and 3 mL of methanol were added to a 50 mL round bottom flask to form a yellow solution. 6M hydrochloric acid (3.5 mL) was added, stirred at room temperature for 6 hours, and TLC detected that the starting compound 3a disappeared. Stop the reaction, neutralize to neutral with 10% sodium carbonate solution, and separate the layers. The aqueous phase was extracted three times with dichloromethane, the organic phases were combined, dried over anhydrous sodium sulfate, and purified by column chromatography to obtain the product 4a as a yellow solid, 480 mg, with a yield of 98%. 1 H NMR (400MHz, CDCl 3 ,298K)δ=11.90(s,1H),9.84(s,1H),8.09(d,J=1.3,1H),8.00(d,J=8.9,1H),7.95(d,J=8.9,1H ),7.91(d,J=7.8,1H),7.69(d,J=2.0,1H),7.63(d,J=2.0,1H),7.44–7.36(m,4H),7.36–7.30(m, 1H), 7.14(t, J=7.9, 2H), 1.44(s, 9H). 13 C NMR (100MHz, CDCl 3 ,298K)δ=196.66,161.18,153.71,152.94,138...
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical purity | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com