Copper ion detection reagent and application
A copper ion detection and copper ion technology, applied in the field of chemical detection, can solve the problems of instrument detection interference, high detection cost, difficult to popularize and apply, etc., and achieve the effects of fast response, simple preparation process and uniform particle size
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
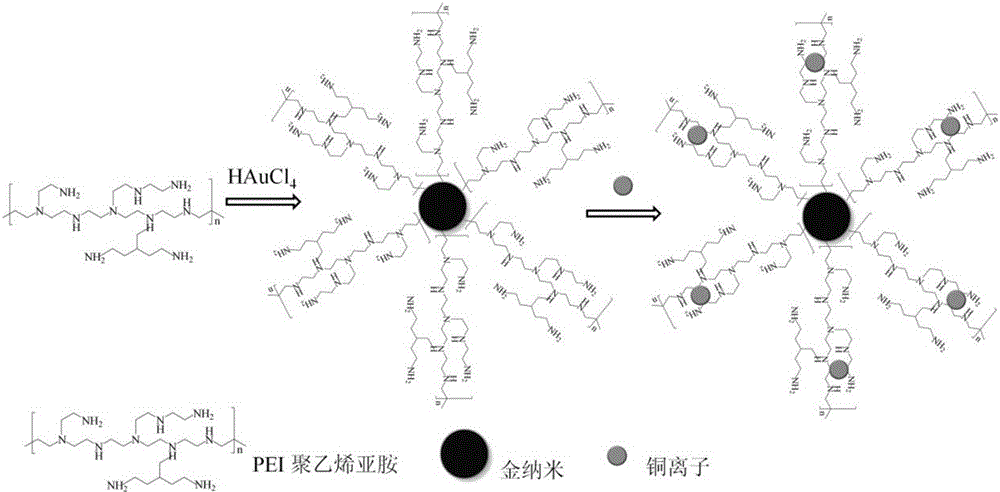
[0021] a. 200μL 242mMHAuCl 4 Add 9100 μL of secondary water, and keep the temperature of the water bath at 65°C; quickly add an appropriate amount of freshly prepared PEI (Mw10000, 10mM) solution to the above solution, stir at a constant speed, and continue to react for 1h until the color becomes stable wine red, in which PEI and HAuCl 4 The ratio is 1:7; after the above solution is cooled to room temperature, centrifuge at 3000r / min for 15 minutes, repeat twice to remove larger particles, and obtain a stable copper ion detection reagent, that is, PEI-modified gold nanoparticles (PEI / AuNPs).
[0022] b. Take 80 μL of the gold nanoparticle solution prepared above and dissolve it in PBS buffer solution (pH 7.4).
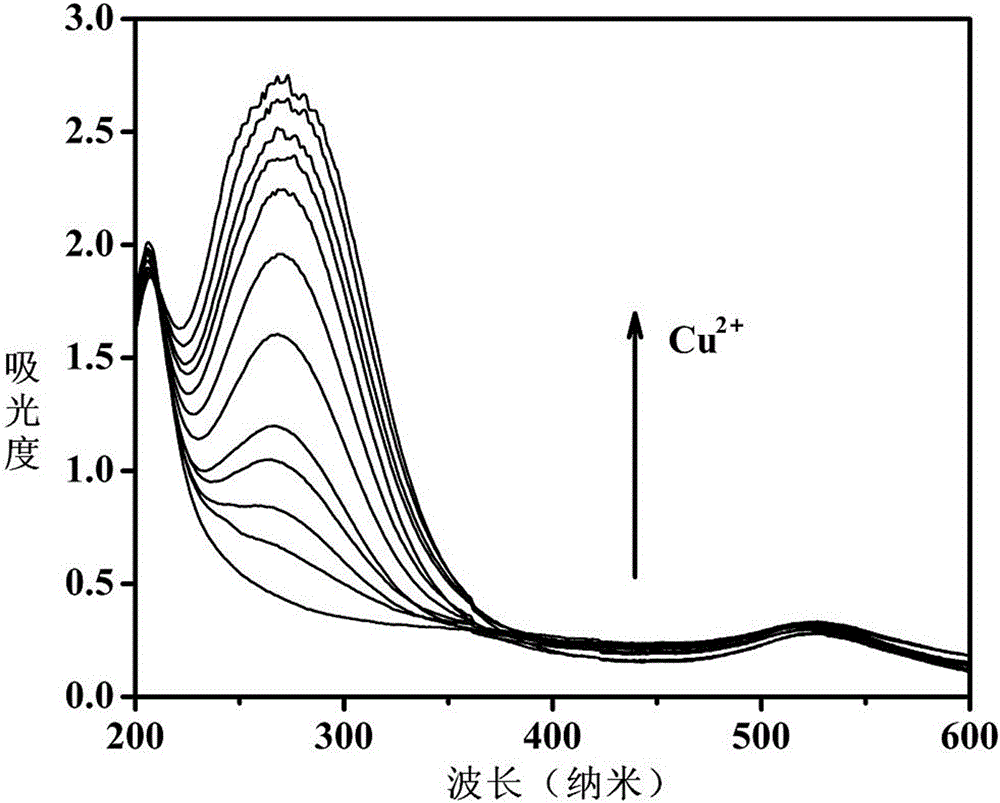
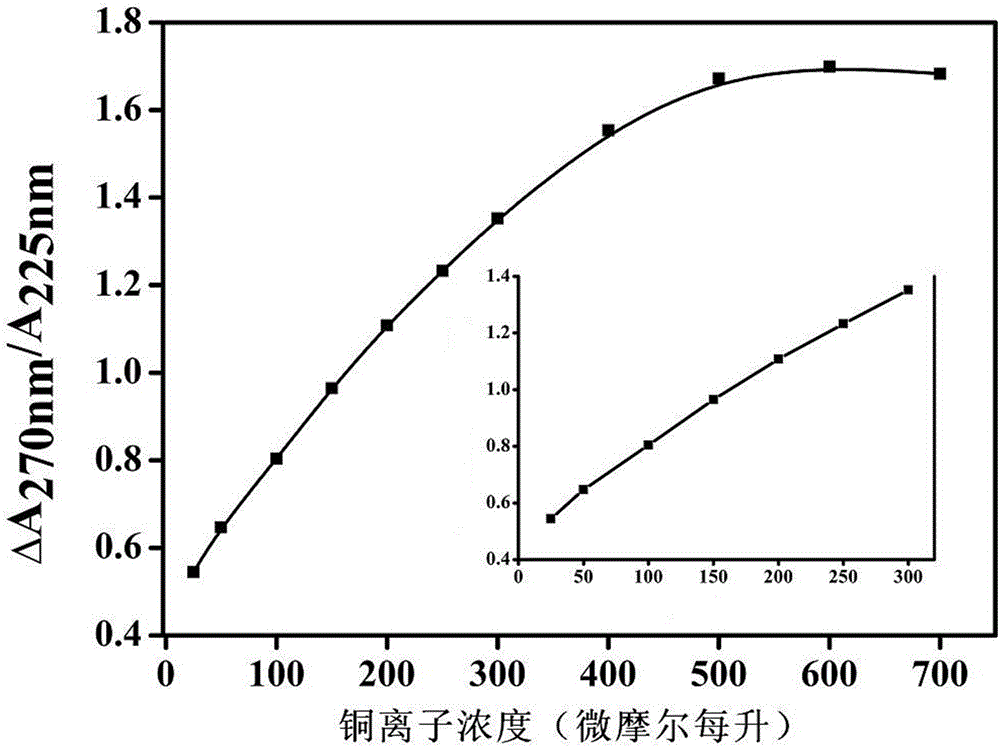
[0023] c. Add Cu to the above system 2+ Standard solution, after incubating for 5 minutes, the reaction is fully carried out before detection, the detection principle is as follows: figure 1 Shown; Take above-mentioned nano-sol, test ultraviolet-visible spectrum.
...
Embodiment 2
[0029] The experimental process and experimental steps are the same as those in Example 1a-c. Cu 2+ The standard solution was replaced with water from Yuquan River of Jiangsu Normal University. Take 15, 62, 125, 220μM Cu respectively 2+ The Yuquan River water of the standard solution was tested, and the calculated standard addition recovery rate was 95%-101%, and the relative standard deviation was 2.2%.
Embodiment 3
[0031] The experimental process and experimental steps are the same as those in Example 1a-c. Cu 2+ The standard solution was replaced with wastewater from a chemical plant (Xuzhou, China). Take 500 μL after diluting 5 times of industrial wastewater for detection, and substitute it into the formula to calculate Cu 2+ The concentration was 162.87μM, which was very close to the detection result of atomic absorption method (168.32μM).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com