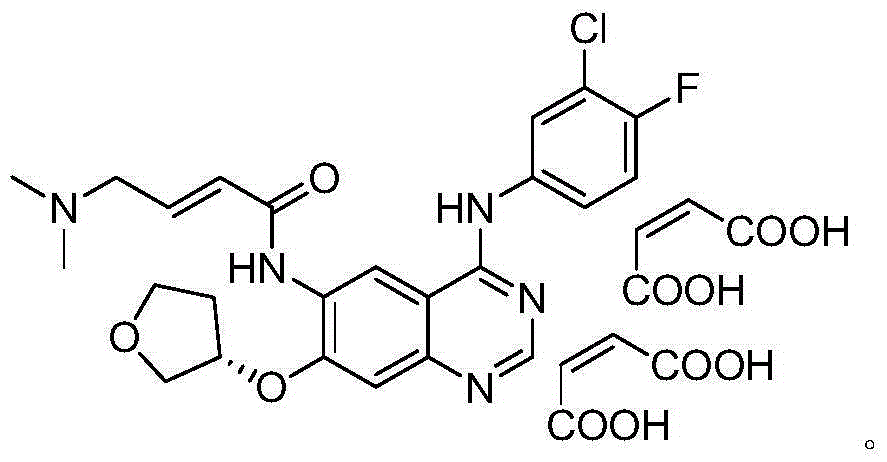
Solid preparation containing BIBW 2992MA2 and preparation method thereof
An intermediate and lubricant technology, applied in the field of medicinal chemistry, can solve the problems of increased pollution of hydrolysis degradation products, poor fluidity and processability, inappropriate content measurement value, etc., and achieves small difference in tablet weight, short production cycle, protection The effect of stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
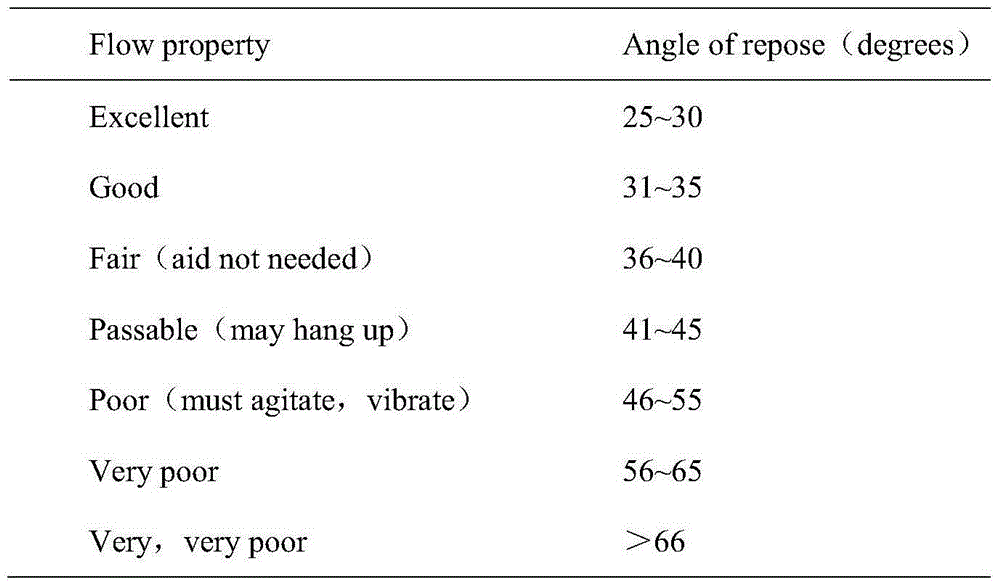
[0058] After crushing and sieving BIBW2992MA2, the fine powder obtained through a sieve of 80 to 120 meshes is mixed with different amounts of flow aids to obtain pretreated powder. The angle of repose of each powder was measured, and the results are shown in Table 3.
[0059] table 3
[0060]
[0061] Note: The amount of glidant is calculated by the weight of BIBW2992MA2.
Embodiment 2
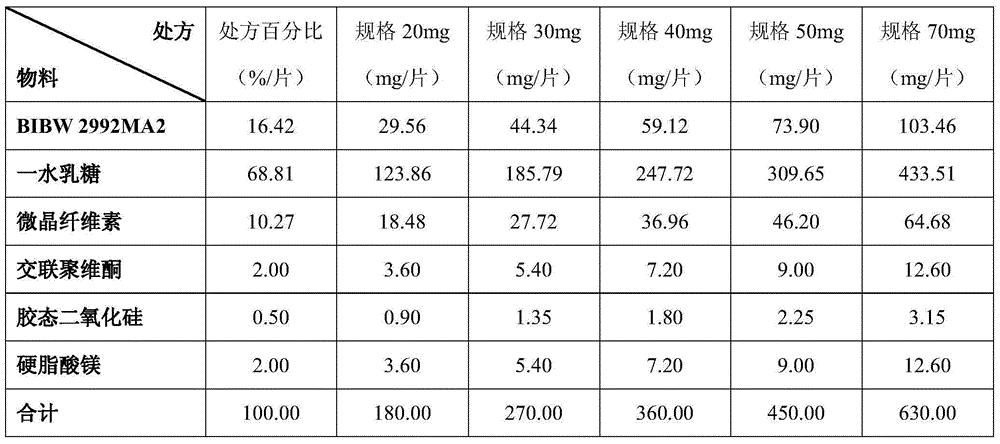
[0063] (1) Prepare tablets containing BIBW2992MA2 according to the composition of the prescription in Table 4. Each prescription is calculated as 500 g of the total mixed powder.
[0064] The BIBW2992MA2 fine powder passed through a 100-mesh sieve was uniformly mixed with colloidal silicon dioxide to obtain a pretreatment powder. The obtained pretreatment powder is uniformly mixed with spray lactose, microcrystalline cellulose and crospovidone, and then uniformly mixed with magnesium stearate to obtain the total mixed powder. Use a rotary tablet press to compress the total mixed powder into tablets, and control the appropriate tableting speed and tableting force so that the difference in tablet weight is less than 0.36g±5%, and the disintegration time of the tablet is less than 15min.
[0065] Table 4
[0066]
[0067] (2) Sample testing
[0068] The angle of repose, tablet weight difference, disintegration time and dissolution rate of each prescription sample were determ...
Embodiment 3
[0072] (1) According to the prescription composition (Table 4) of Example 2, a tablet containing BIBW2992MA2 was prepared by feeding, and each prescription was calculated as 500 g of the total mixed powder.
[0073] Take the BIBW2992MA2 fine powder passed through the 80-mesh sieve and mix it evenly with colloidal silicon dioxide to obtain the pretreatment powder. The obtained pretreatment powder is uniformly mixed with spray lactose, microcrystalline cellulose and crospovidone, and finally mixed with magnesium stearate to obtain the total mixed powder. Use a rotary tablet press to compress the total mixed powder into tablets, and control the appropriate tableting speed and tableting force so that the difference in tablet weight is less than 0.36g±5%, and the disintegration time of the tablet is less than 15min.
[0074] (2) Sample testing
[0075] The angle of repose, tablet weight difference, disintegration time and dissolution rate of each prescription sample were determine...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com