Aristolochane-type sesquiterpenoids and their preparation method and application
A technology of aristolochane and sesquiterpenoids, which is applied to the effective components of hydroxyl compounds, the separation/purification of hydroxyl compounds, the separation/purification of carbonyl compounds, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
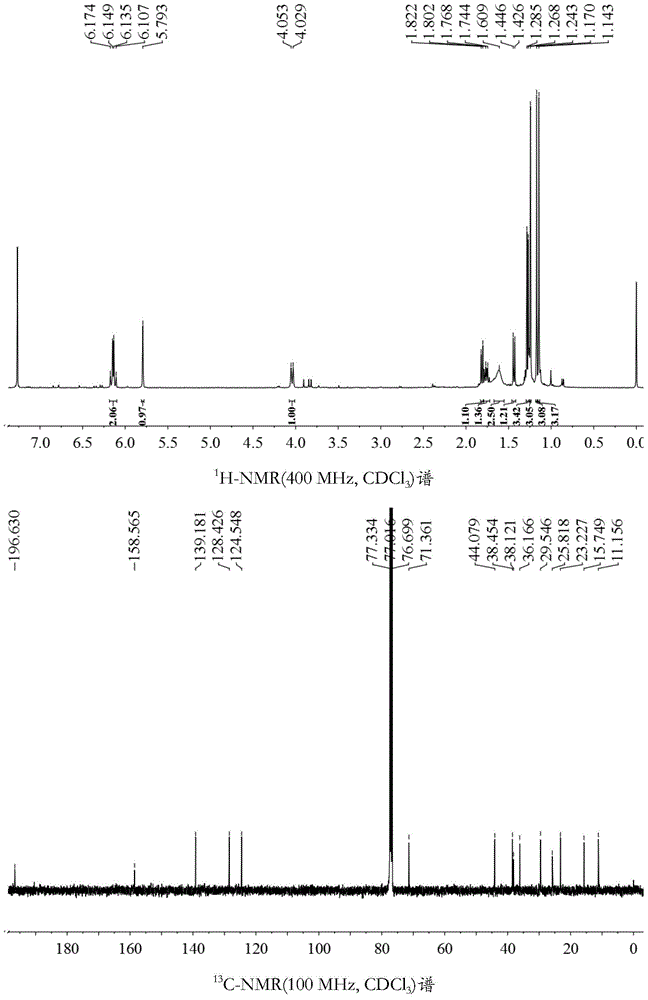
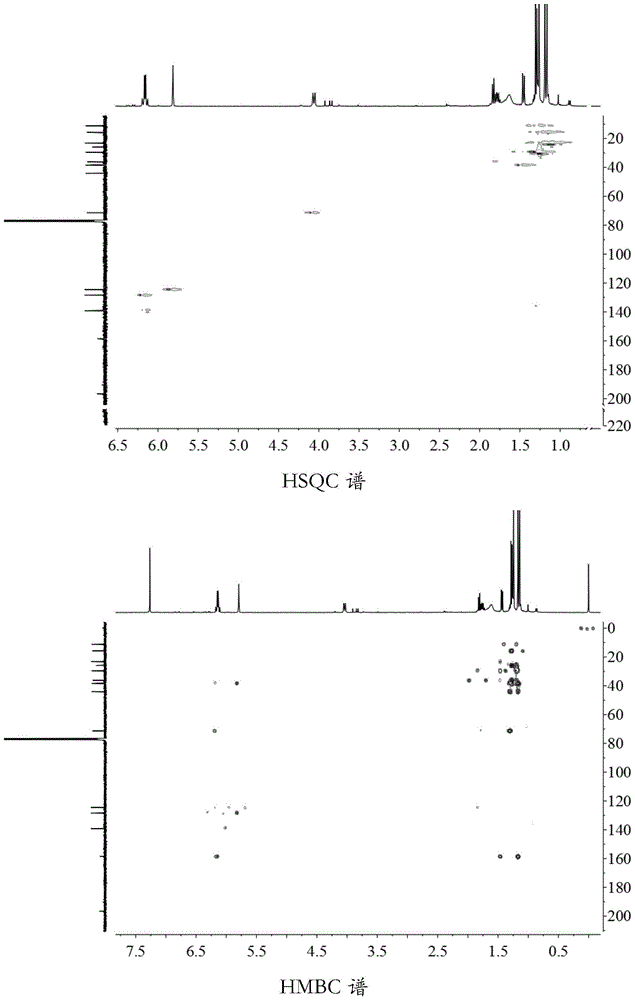
[0049] Preparation of the active ingredient aristolochane-type sesquiterpene and its derivatives (extraction and separation process):
[0050]The decoction pieces of Gansong medicinal materials were purchased from Anhui Jiren Pharmaceutical Co., Ltd. (batch number: 110709, specification: 1kg / bag, place of origin: Sichuan), about 20kg, and 20kg of Gansong rhizome was extracted with 70% ethanol for 3 times, each time for 48 hours. Combine the extracts, concentrate under reduced pressure to obtain 3 kg of crude extract extract; then heat extract the above-mentioned medicinal residues with 70% ethanol for 3 times, each time for 2 hours, combine the extracts, concentrate under reduced pressure, and obtain 400 g of crude extract extract; Combine the two crude extracts to obtain 3.4 kg of total extract; the total extract of the obtained crude extract, after being dispersed in water, is sequentially extracted with an equal volume of petroleum ether, ethyl acetate, and n-butanol to obta...
Embodiment 2
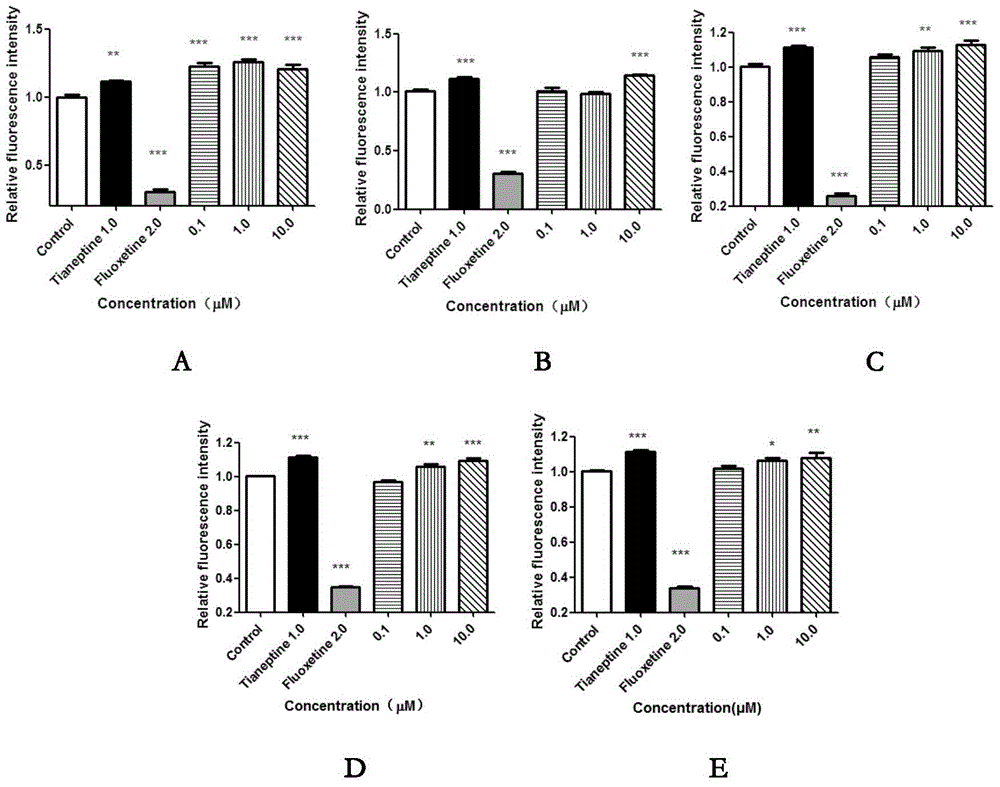
[0066] Effects of Compounds of the Invention on Serotonin Transporter (SERT)
[0067] Using stably transfected hSERT-HEK293 cell line, 4-(4-(dimethylamino)phenyl)-1-methylpyridinium (APP + ) is a fluorescent substrate, and the effect of the aristolochane-type sesquiterpene and its derivatives on SERT activity was detected on a high-content system.
[0068] 1) Experimental instruments and reagents
[0069] laboratory apparatus:
[0070] High-content Operetta system and Columbus data management and analysis system (PerkinElmer), ultra-clean bench, pipette gun (1000μL, 200μL, 20μL, 10μL, 2.5μL, Eppendorf, USA)
[0071] Reagents and materials:
[0072] Human embryonic kidney cell line HEK293 (Cell Bank of Type Culture Collection Committee, Chinese Academy of Sciences), hSERT pcDNA3 plasmid (Addgene, plasmid 15483), MEM medium (Gibco), APP + (Sigma), Hoechst 33342 (CellSignaling Technology), 96-well plate (Costar 3605)
[0073] 2) Experimental operation process
[0074] First...
Embodiment 3
[0097]
[0098] Preparation method: uniformly mix aristolochne-9β-alcohol / nabisone / nabisone H / 3-hydroxynabisone H / 3-oxonabisone H, lactose and starch according to the above ratio, and pass 200 Mesh sieve, wet evenly with water, dry the wetted mixture and then sieve, add magnesium stearate, then compress the mixture into tablets, each tablet weighs 250mg, and the active ingredient content is 10mg.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com