Automatic preparation method and device for <18>F-(2S,4R)-4-fluoro-L-glutamine
A technology for glutamine and fluorination reaction, which is applied in the field of automatic preparation of 18F-(2S,4R)-4-fluoro-L-glutamine and its device, and can solve the problems of inapplicability, difficult contact, and corrosion resistance of materials. Sexual issues, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0112] Embodiment 1 (carried out intermediate purification by reverse-phase purification cartridge):
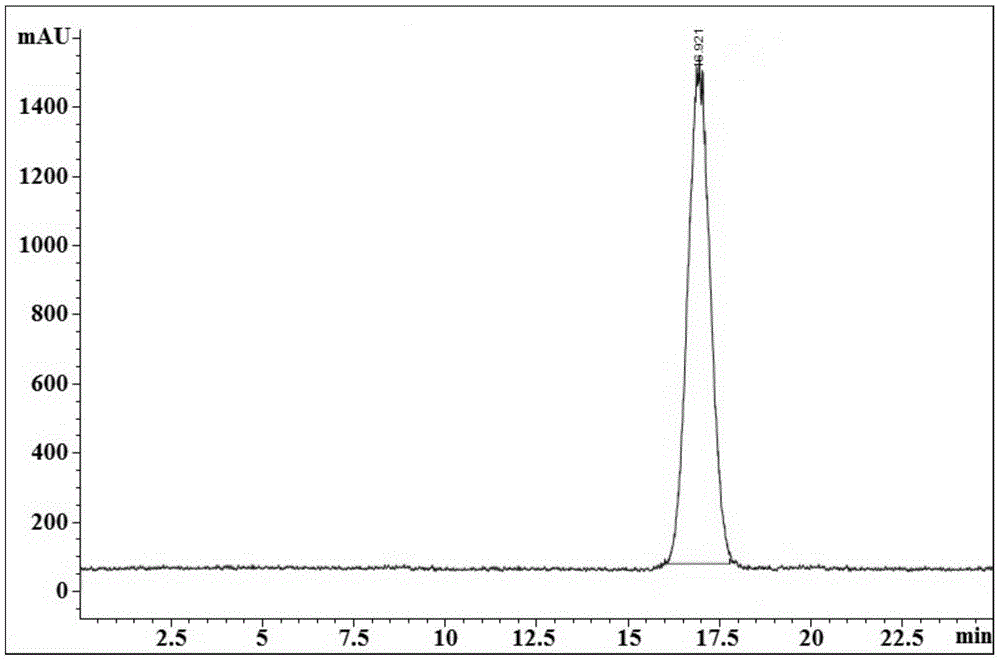
[0113] 18 Preparation of F-(2S,4R)-4-fluoro-L-glutamine.
[0114] Items and materials:
[0115] Contains H 18 The aqueous solution of F was prepared by the Cyclone-10 medical cyclotron accelerator of IBA Company, with a radioactivity of 29.6GBq; 18-crown-6, KHCO 3 , acetonitrile, ethanol, trifluoroacetic acid, anisole (which is used to prepare the trifluoroacetic acid solution containing 0.5% anisole), copper sulfate pentahydrate and other chemical reagents were purchased from Sigma-Aldrich Company and Beijing Chemical Plant, purity All are reagent grade, and used directly without instructions; anion exchange SpePakQMA column, solid phase extraction SpePakC-18 column, OasisHLB column, ion adsorption alumina column were purchased from Waters; SAX strong anion exchange column was purchased from Alltech; sterile Filter membranes were purchased from Merck Millipore.
[0116]...
Embodiment 2
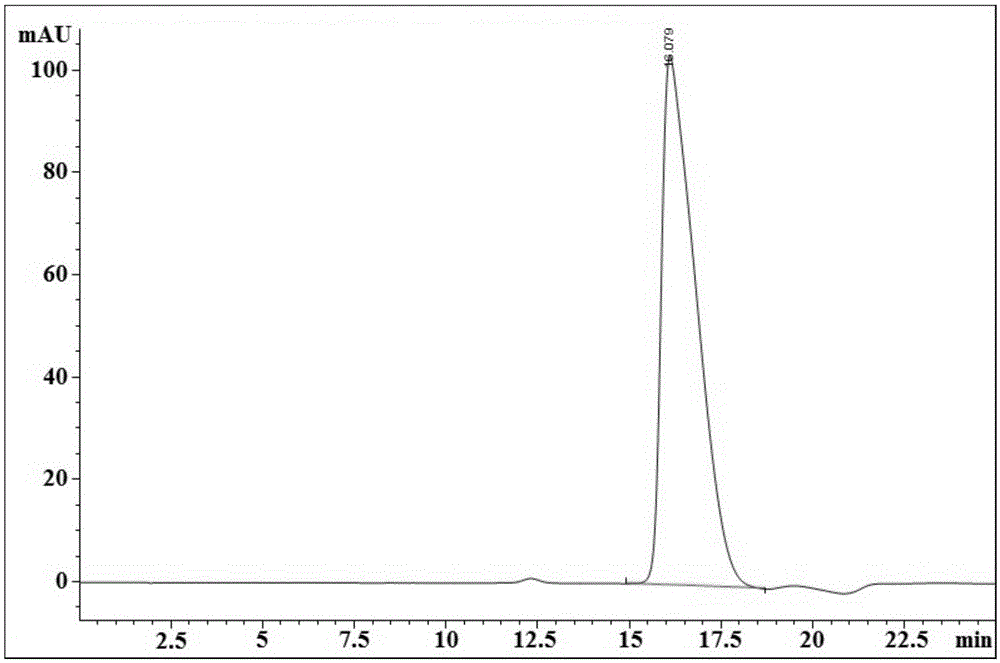
[0146] Example 2 ( 18 F-(2S,4R)-4-fluoro-L-glutamine automatic preparation process optimization)
[0147] use 18 Optimization of automatic preparation device for F-(2S,4R)-4-fluoro-L-glutamine 18 Preparation process of F-(2S,4R)-4-fluoro-L-glutamine.
[0148] improved 18 Phase transfer catalyst 18-crown-6 / KHCO in the preparation process of F-(2S,4R)-4-fluoro-L-glutamine 3 Solution drying process, precursor compound dosage and final product purification method optimization.
[0149] Phase transfer catalyst 18-crown-6 / KHCO 3 The drying of the solution is carried out by the azeotropic evaporation of acetonitrile and water. The number of repeated additions of acetonitrile and evaporation affects the drying effect, and the drying process takes a long time, which has a great impact on the production yield.
[0150] The optimization method is:
[0151] 1) Evaporate and dry once: use 18-crown-6 / KHCO in device vial 1 3 (It can also be amino polyether K 222 / K 2 CO 3 solution...
Embodiment 3
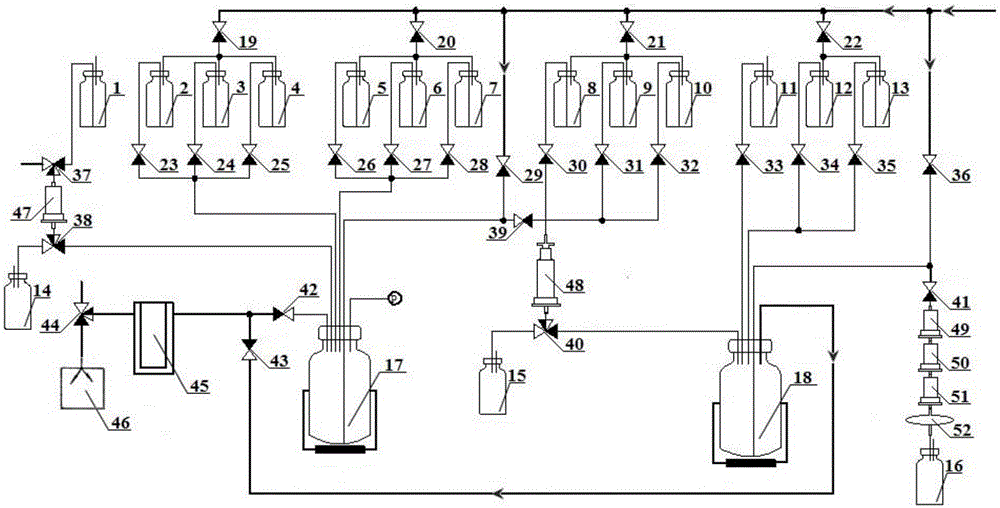
[0161] preparation 18 The purification of intermediates in the F-(2S,4R)-4-fluoro-L-glutamine process can be implemented by high performance liquid chromatography, and the semi-preparative high-pressure liquid chromatography system (HPLC) used is the Belgian IBA company's Synthera HPLC system. The SyntheraHPLC system can be loaded with two mobile phases and contains an online UV detector and an online radioactivity detector. The connection method is as follows: connect the outlet end of the electromagnetic valve 39 of the synthesis device with the sample loop of the HPLC system, and connect the outlet of the mobile phase collected by the HPLC to the vial 8 .
[0162] preparation 18 The preparation work of F-(2S,4R)-4-fluoro-L-glutamine is as follows: connect the normally open end liquid inlet of solenoid valve 37 with that of Cyclone-10 type medical cyclotron 18 f - The solution is exported to the pipeline connection; the gas inlet shared by the device electromagnetic valv...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com