Multiple target point type Tamibarotene derivative as well as preparation method and application thereof
A tamibarotene, multi-target technology, applied in the preparation of urea derivatives, the preparation of organic compounds, chemical instruments and methods, etc., can solve the problems of low drug resistance, toxic and side effects that limit clinical use, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
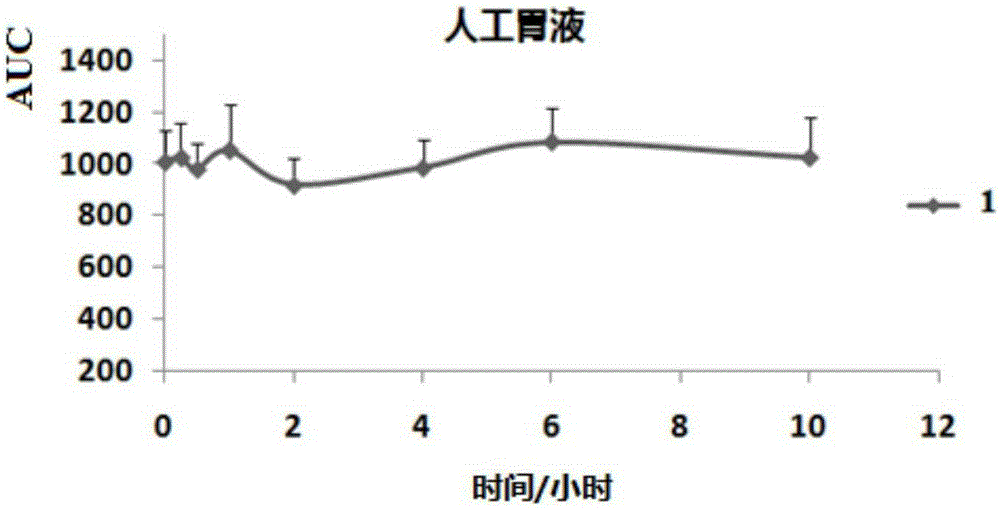
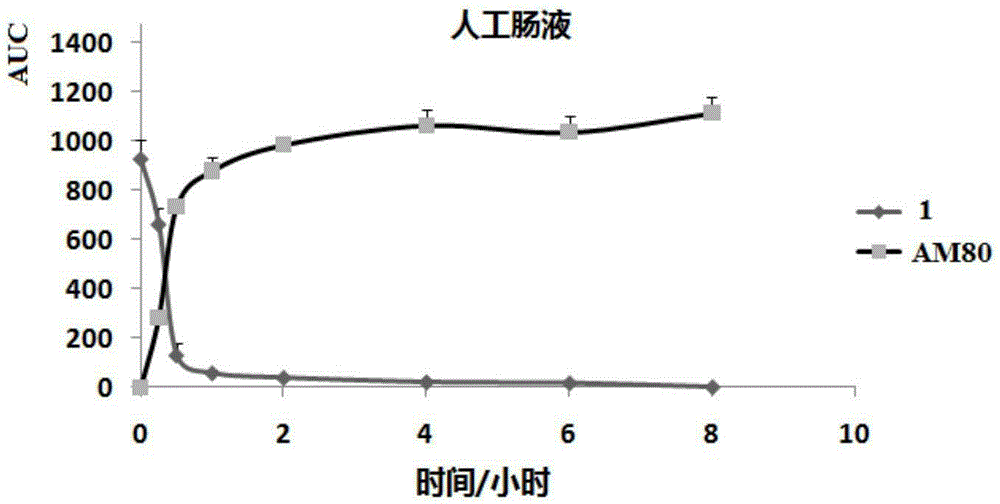
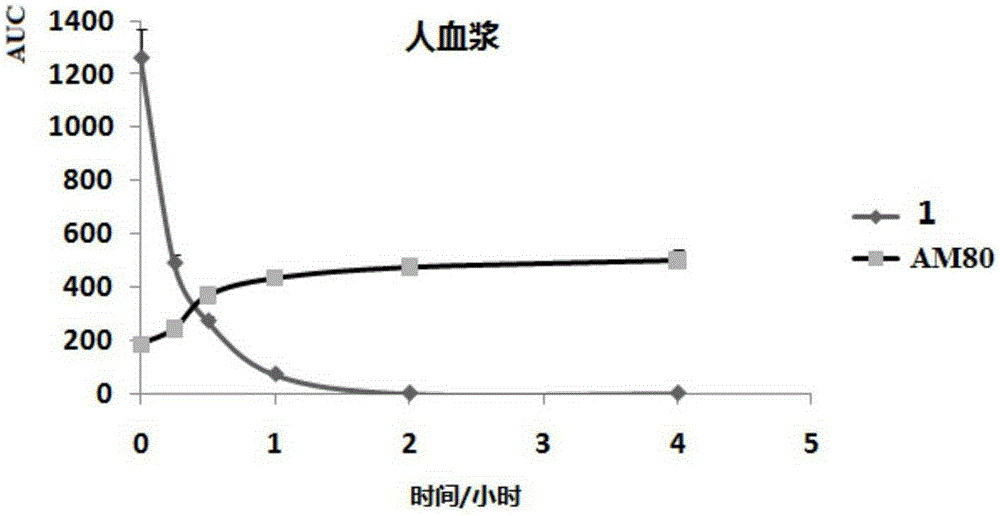
Image
Examples
Embodiment 1
[0063] Example 1: N-(5,5,8,8-tetramethyl-5,6,7,8-tetrahydro-2-naphthyl)-4-((ureaoxy)carbonyl)benzamide ( 1) Preparation
[0064] Tamibarotene (AM80) (0.35g, 1mmol) was dissolved in anhydrous tetrahydrofuran, and 1-ethyl-(3-dimethylaminopropyl)carbodiimide hydrochloride (0.21 g, 1.1mmol), adding pentafluorophenol (0.20g, 1.1mmol) dissolved in N,N-dimethylformamide in advance. The reaction was carried out at room temperature for 12 h, and the reaction was monitored by TLC. After the reaction was completed, the solvent was distilled off for further use. First, hydroxyurea (0.08g, 1mmol) was dissolved in N,N-dimethylformamide, N-methylmorpholine (12mL) was added to dissolve the upper pentafluorophenol ester in anhydrous dioxane, Drop into the N,N-dimethylformamide reaction solution. Reaction at room temperature for 12h. Directly passed through column (EA / PE=5 / 1) to obtain white solid 1 (0.11 g, 27%). ESI-MSm / z:410.5(M+H) + , 1 H-NMR (600MHzDMSO-d 6 ): δ1.24-1.25(m,12H), 1...
Embodiment 2
[0065] Example 2: (5-fluoro-2,4-dioxy-3,4-dihydropyrimidin-1(2H)-yl)methyl-4-((5,5,8,8-tetramethyl Preparation of -5,6,7,8-tetrahydro-2-naphthyl)carbamoyl)benzoate (3)
[0066] 5-Fluorouracil (5-FU) (1.3g, 10mmol) was mixed with 3mL of 37% by volume formaldehyde solution, stirred at 60°C for 2h, and evaporated under reduced pressure to obtain a colorless viscous oil. The obtained oil was dissolved in anhydrous acetonitrile, and AM80 (3.51g, 10mmol), dicyclohexylcarbodiimide (2.3g, 11mmol), and an appropriate amount of 4-dimethylaminopyridine were added. After 24 hours, TLC monitored that the reaction was complete, evaporated the solvent, added ethyl acetate to dissolve, and washed the organic phase with water, 1M hydrochloric acid, saturated sodium bicarbonate, and saturated sodium chloride, respectively. After drying with anhydrous magnesium sulfate, the solvent was evaporated by filtration, and the residue was separated by silica gel column (dichloromethane / methanol 60:1) t...
Embodiment 3
[0067] Example 3: N 1 -(2-(2,6-Piperidinedione-3-yl)-1-oxoisoindoline-4-yl)-N 4 Preparation of (5,5,8,8-tetramethyl-5,6,7,8-tetrahydro-2-naphthyl)terephthalamide (5)
[0068] Tamibarotene (AM80) (0.35 g, 1 mmol) was dissolved in 20 mL of thionyl chloride, two drops of DMF was added dropwise, and stirred and refluxed at 75° C. for 2 h. The solvent was distilled off to obtain compound 4 as a yellow oil. Lenalidomide (0.26 g, 1 mmol) was dissolved in pyridine solution, and compound 4 in tetrahydrofuran was added dropwise under ice-cooling. Remove from the ice room and warm the reaction. After 24 hours, TLC monitored that the reaction was complete. The solvent was evaporated, ethyl acetate was added for dissolution, and the organic phase was washed with water, 1M hydrochloric acid and saturated sodium chloride respectively. After drying with anhydrous magnesium sulfate, the solvent was evaporated by filtration, and the residue was separated on a silica gel column (petroleum et...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com