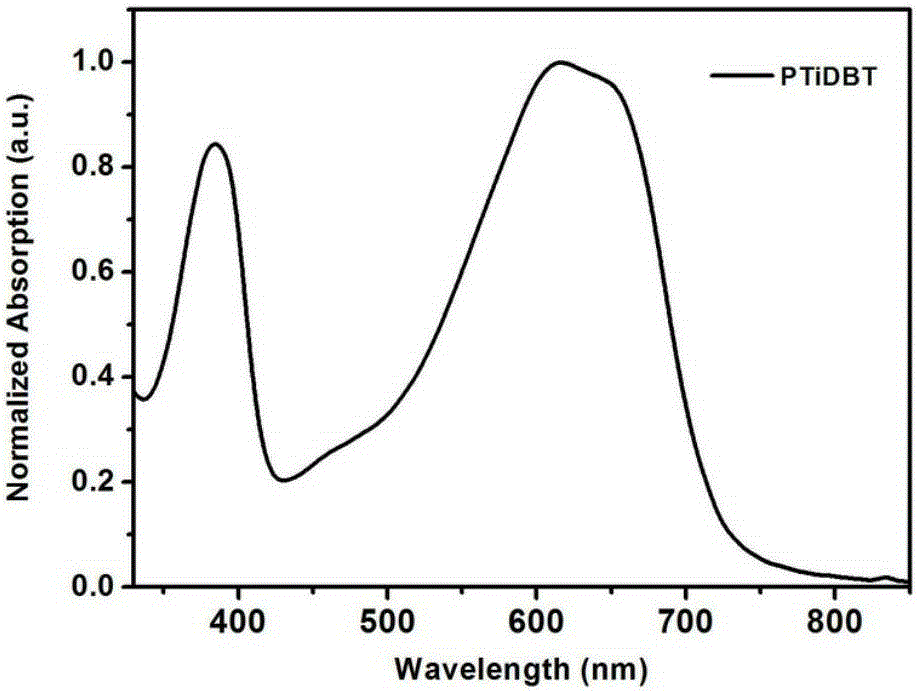
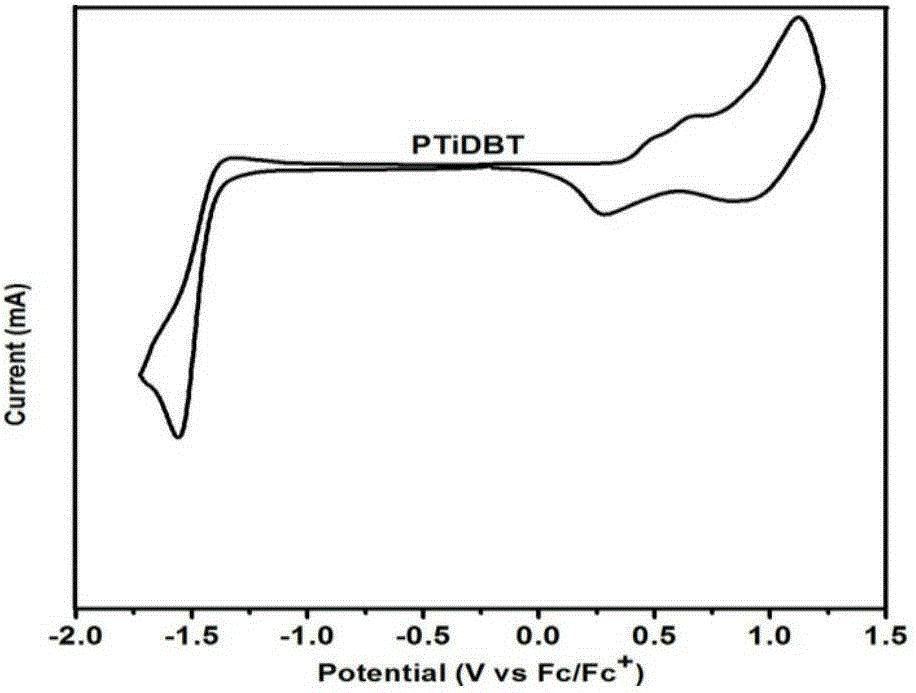
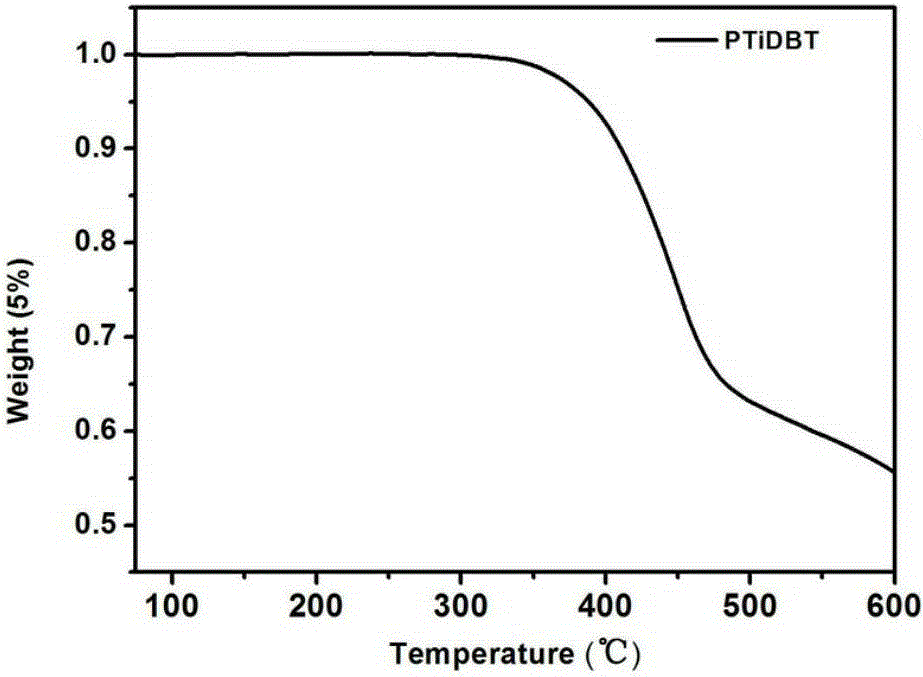
Conjugated polymer semiconductor materials based on thienoarylindole unit and preparation method thereof, and application of conjugated polymer semiconductor materials in high-efficiency polymer solar cells.
A technology of conjugated polymers and semiconductors, which is applied in the manufacture of semiconductor/solid-state devices, semiconductor devices, circuits, etc., can solve the problems of application research in the ascendant, and achieve the effects of good solubility, convenient purification, and simple preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Preparation of Polymer PTiDBT(I)
[0049] In a single-necked round bottom flask (250mL), add p-dibromobenzene (11.8g, 50mmol), dichloromethane (50mL), under ice-water bath, add nitric acid (10mL) and sulfuric acid (27.5mL) dropwise, at 0°C , stirred for 2 hours, then added water to quench the reaction, extracted with dichloromethane, washed the organic phase with water, saturated sodium bicarbonate, and saturated sodium chloride solution, dried over anhydrous magnesium sulfate, concentrated, and recrystallized from ethanol to obtain 12.6g of 2-nitro - p-dibromobenzene, yield 90%.
[0050]
[0051] In a two-neck flask (100mL), dissolve 2-nitro-p-dibromobenzene (2.80g, 10mmol), 2-thiopheneboronic acid (1.53g, 12mmol) in 20mL of toluene, inject 15mL of 2M potassium carbonate solution. Gas three times, under the protection of nitrogen, add tetrakis(triphenylphosphine)palladium (150mg, 0.14mmol), heat to 75°C, react for 24 hours. Naturally cooled to room temperature, ex...
Embodiment 2
[0065] Preparation of Polymer PTiDDTBT(II)
[0066] In a single-necked round-bottomed flask (250mL), add p-dibromobenzene (12.0g, 50mmol), dichloromethane (50mL), add nitric acid (10mL) and sulfuric acid (25mL) dropwise under an ice-water bath, and at 0°C, Stir the reaction for 2 hours, then quench the reaction with water, extract with dichloromethane, wash the organic phase with water, saturated sodium bicarbonate, and saturated sodium chloride solution, dry over anhydrous magnesium sulfate, concentrate, and recrystallize from ethanol to obtain 12.5 g of 2-nitro- For p-dibromobenzene, the yield is 90%.
[0067]
[0068] In a two-neck flask (100mL), dissolve 2-nitro-p-dibromobenzene (2.80g, 10mmol), 2-thiopheneboronic acid (1.42g, 11mmol) in 30mL of toluene, inject 20mL of 2M potassium carbonate solution. Gas three times, under the protection of nitrogen, add tetrakis(triphenylphosphine)palladium (150mg, 0.14mmol), heat to 90°C, react for 24 hours. Naturally cooled to roo...
Embodiment 3
[0082] Preparation of Polymer PTiDTBT(II)
[0083] In a two-neck flask (150mL), dissolve the alkylated thienoindole (4.62g, 10mmol), 2-thiophene boronic acid (1.42g, 11mmol) in 50mL of toluene, inject 30mL of 2M potassium carbonate solution. Three times, under nitrogen protection, tetrakis(triphenylphosphine)palladium (150mg, 0.14mmol) was added, heated to 90°C, and reacted for 24 hours. Naturally cooled to room temperature, extracted with dichloromethane, dried, concentrated, and separated by column chromatography to obtain 3.75 g of a colorless liquid with a yield of 78%.
[0084] 1 HNMR (400MHz, CDCl 3 )δ (ppm) 7.73 (d, J = 8.2Hz, 1H), 7.59 (d, J = 1.2Hz, 1H), 7.45 (dd, J = 8.2, 1.5Hz, 1H), 7.40–7.33 (m, 2H ),7.28(d,J=1.1Hz,1H),7.11(dd,J=5.1,3.5Hz,1H),7.04(d,J=5.2Hz,1H),4.14(d,J=7.3Hz,2H ),2.39-1.95(m,1H),1.43-1.19(m,24H),0.86(m,6H). 13 CNMR (100MHz, CDCl 3 )δ(ppm)146.57,146.03,142.08,128.84,128.00,126.92,124.03,122.43,121.25,119.19,117.76,115.95,110.68,107.37,49.67,3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com