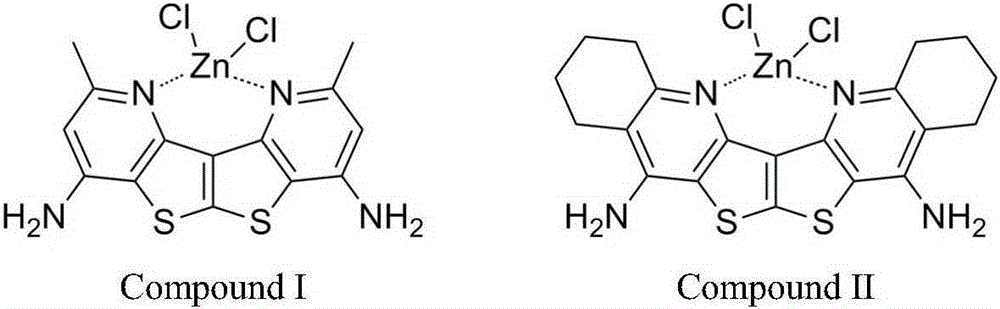
Dithienopyridine zinc coordination compound luminescence material and preparation method thereof
A technology of phenopyridine zinc and luminescent materials, which is applied in the direction of luminescent materials, zinc organic compounds, chemical instruments and methods, etc., can solve the problems of insufficient simplification of the production process, short device life, and low product share, and achieve structural stability, High atom economy and wide-ranging effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example example 1
[0031] Example 1 The preparation process of bisthiophene pyridine zinc complex luminescent material I
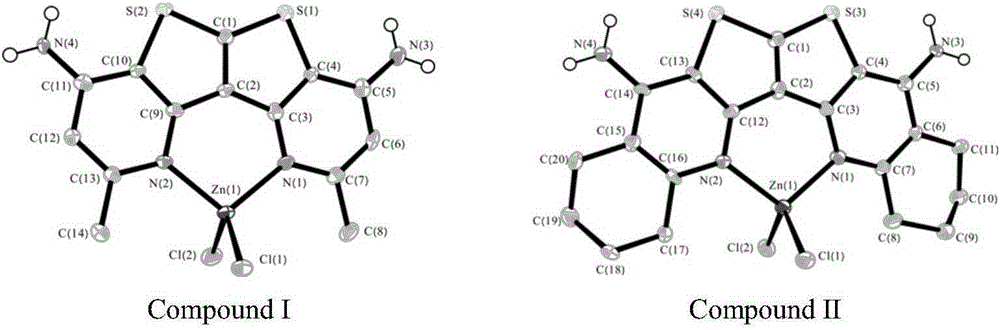
[0032] Add 3,4-diaminothieno[2,3-b]thiophene-2,5-dinitrile (1mmol) and acetone (4mL) to a 12mL polytetrafluoroethylene liner in a hydrothermal reactor, and stir until evenly dispersed, add ZnCl 2 (5mmol), the polytetrafluoroethylene liner was reacted at 140°C for 3.0h. After the reaction, directly filter and wash with water. The crude product was recrystallized with ethanol and water to obtain pale yellow crystals (I), and the single crystal structure of complex I was as follows: figure 2 a, the yield is about 60% (mother liquor recovery), m.p.>300°C. The reaction formula of 3,4-diaminothieno[2,3-b]thiophene-2,5-dinitrile and acetone is:
[0033]
[0034] The spectral data of product (I) is 1 HNMR (DMSO-d 6 ,400MHz)δ:7.55(s,4H,NH 2 ),6.58(s,2H,ArH),2.72(s,6H,CH 3 ). 13 CNMR (DMSO-d 6 ,100MHz) δ: 155.7, 151.9, 146.7, 146.0, 129.7, 119.0, 104.1, 22.8.
example example 2
[0035] Example 2 The preparation process of bisthiophene pyridine zinc complex luminescent material II
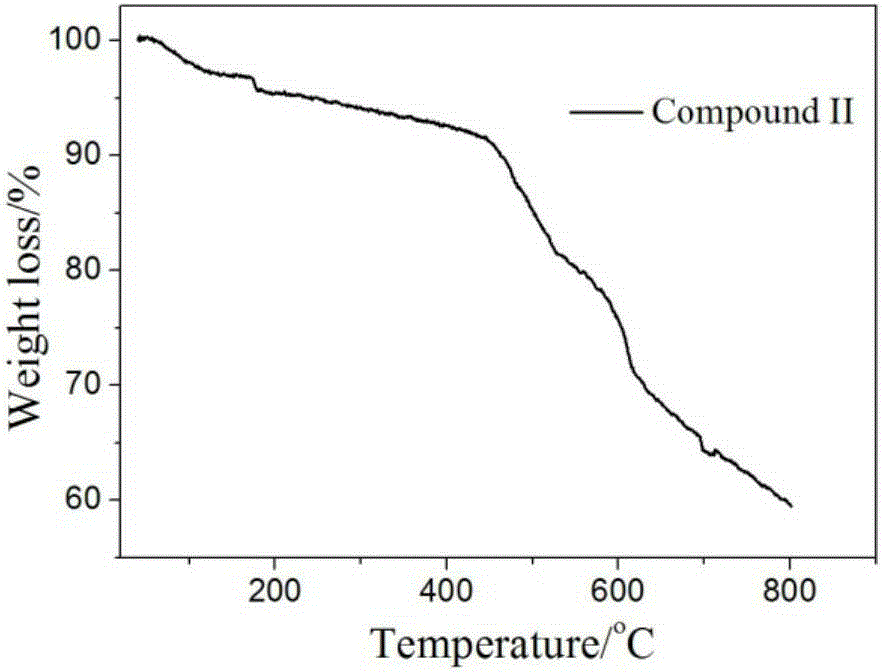
[0036] Add 3,4-diaminothieno[2,3-b]thiophene-2,5-dicarbonitrile (1mmol) and cyclohexanone (4mL) into a 25ml one-necked flask, stir until uniformly dispersed, add ZnCl 2 (5 mmol), and then rapidly heated to reflux at 150° C., and reacted with magnetic stirring for 4 h. After the reaction, directly filter and wash with water to obtain a solid mixture. The crude product was recrystallized from a mixture of DMF and water to obtain white crystals (II), and the single crystal structure of complex I was as follows: figure 2 a, the yield is about 74% (mother liquor recovery), m.p.>300°C. Measure its thermal weight, such as image 3 shown. The reaction formula of 3,4-diaminothieno[2,3-b]thiophene-2,5-dicarbonitrile and cyclohexanone is:
[0037]
[0038] The spectral data of product (II) is: 1 HNMR (DMSO-d 6 ,400MHz)δ:7.25(s,4H,NH 2 ),3.27(s,4H,CH 2 ),2.57(s,4H,CH 2 ), 1...
example example 3
[0039] Fluorescence test of example 3 bisthiophene pyridine zinc complexes
[0040] The bisthiophene pyridine zinc complex prepared in Example 2 was dissolved in DMSO and prepared as 1*10 -2 mol / L stock solution. Take 3uL from the stock solution and add it to 3mL water to prepare 1*10 -5 mol / L ethanol solution, the fluorescence emission performance test was carried out on a Hitachi F-7000 fluorescence spectrometer.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com