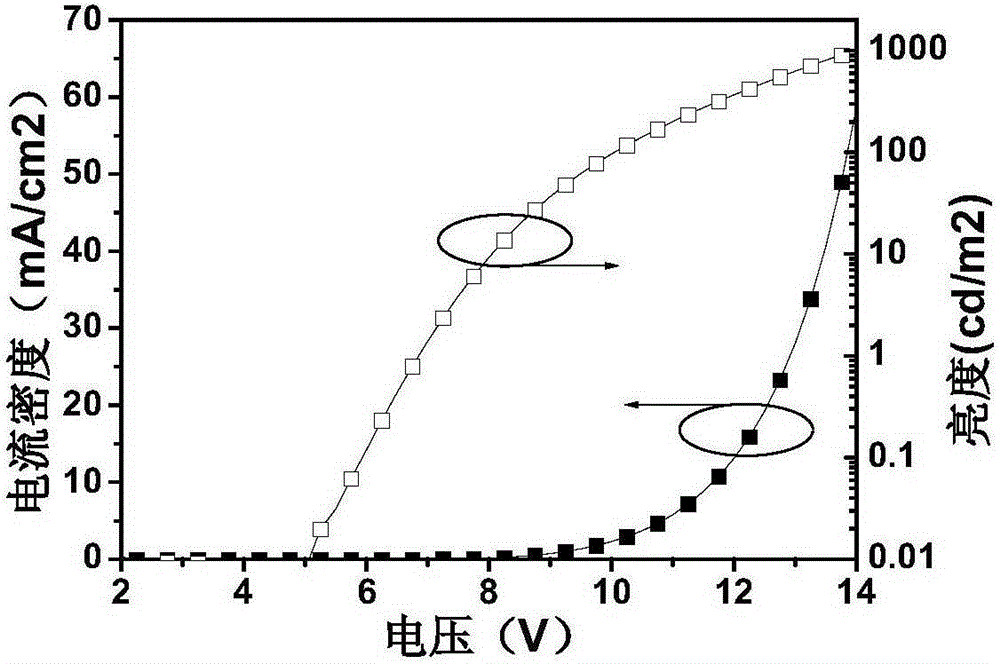
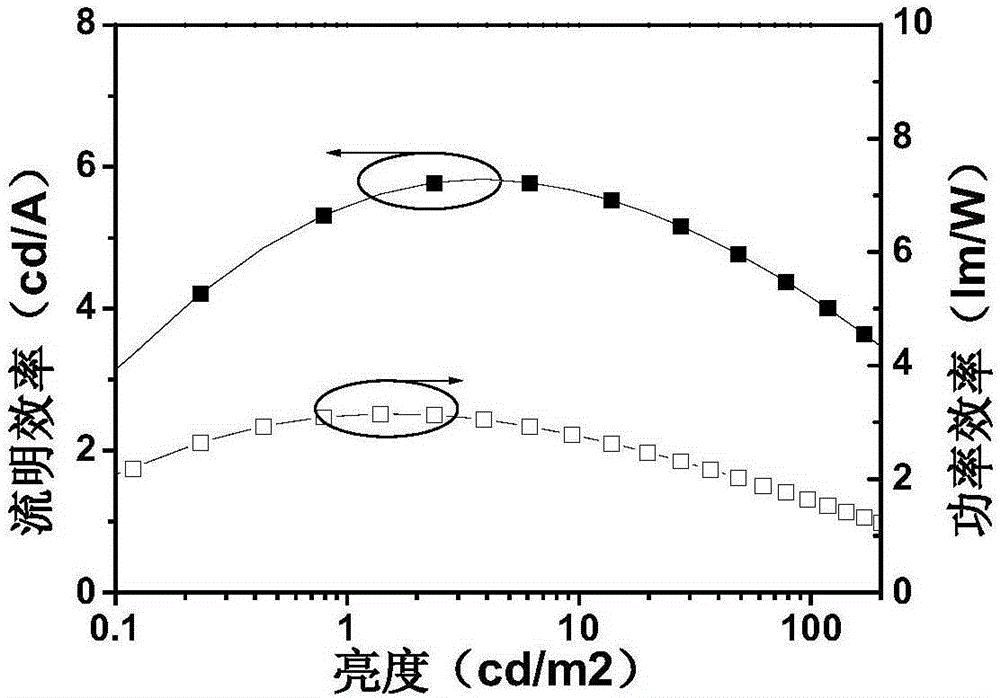
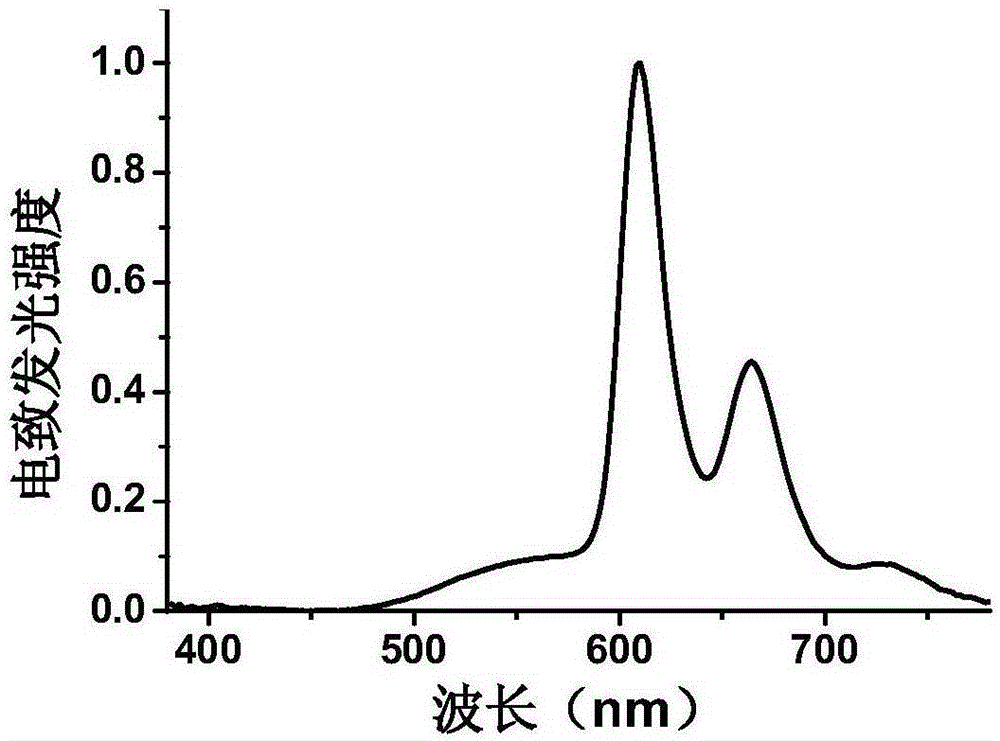
Preparation method and application of 4,4'-substituted benzil core-based luminescence and host material
A technology of host material and benzil nucleus, which is applied in the field of preparation and application of luminescence and host materials, can solve problems such as the potential of organic light-emitting diodes to be tapped, and achieve the effects of improving external quantum efficiency, good solubility, and single structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] In this example, the luminescent and host material P1 based on 4,4'-substituted benzil nucleus is prepared, and the reaction is shown in the following formula:
[0036]
[0037] Specific implementation steps: add 4,4'-dibromo-substituted benzil (0.368mg, 1mmol) and carbazole (367.4mg, 2.2mmol) and potassium carbonate (552mg, 4mmol) to dry toluene in a 250ml three-necked flask, Palladium acetate (44mg, 0.2mmol) was added under nitrogen protection, and then tri-tertbutylphosphine (0.73ml, 0.73mmol) was added dropwise thereto. Under the protection of nitrogen, the reaction was heated to reflux for 12h. After the reaction was completed, it was cooled to room temperature, extracted with dichloromethane, washed with water, dried over anhydrous magnesium sulfate and passed through a silica gel column to obtain 500 mg of a yellow solid (90% yield). Molecular formula: C 38 h 24 N 2 o 2 ; Molecular weight: 540.62; Elemental analysis: C, 84.42; H, 4.47; N, 5.18; O, 5.92. ...
Embodiment 2
[0039] In this example, the luminescent and host material P2 based on 4,4'-substituted benzil nucleus is prepared, and the reaction is shown in the following formula:
[0040]
[0041] Specific implementation steps: in a 250ml three-necked flask, add 4,4'-dibromo-substituted benzil (0.368mg, 1mmol) and tert-butylcarbazole (614mg, 2.2mmol) and potassium carbonate (552mg, 4mmol) to dry toluene , palladium acetate (44mg, 0.2mmol) was added under nitrogen protection, and then tri-tertbutylphosphine (0.73ml, 0.73mmol) was added dropwise thereto. Under the protection of nitrogen, the reaction was heated to reflux for 12h. After the reaction was completed, it was cooled to room temperature, extracted with dichloromethane, washed with water, dried over anhydrous magnesium sulfate, and passed through a silica gel column to obtain 700 mg of a yellow solid (91.5% yield). Molecular formula: C 54 h 56 N 2 o 2 ; Molecular weight: 765.05; Elemental analysis: C, 84.78; H, 7.38; N, 3.6...
Embodiment 3
[0043] In this example, the luminescent and host material P3 based on 4,4'-substituted benzil nucleus is prepared, and the reaction is shown in the following formula:
[0044]
[0045] Specific implementation steps: under a nitrogen atmosphere, add 100ml of toluene, 30ml of absolute ethanol, 40ml of 2M potassium carbonate aqueous solution, 4,4'-dibromo-substituted benzil (0.368mg, 1mmol), p-carbazole to a 250ml flask Phenylboronate (811mg, 2.2mmol), and then 200mg of triphenylphosphopalladium catalyst was added, and the mixture was refluxed at 110°C for 12 hours. Cool to room temperature, extract with dichloromethane, and dry over anhydrous magnesium sulfate. Column chromatography separated 600 mg of yellow solid with a yield of 86.7%. Molecular formula: C 50 h 32 N 2 o 2 ; Molecular weight: 692.82; Elemental analysis: C, 86.68; H, 4.66; N, 4.04; O, 4.62.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com