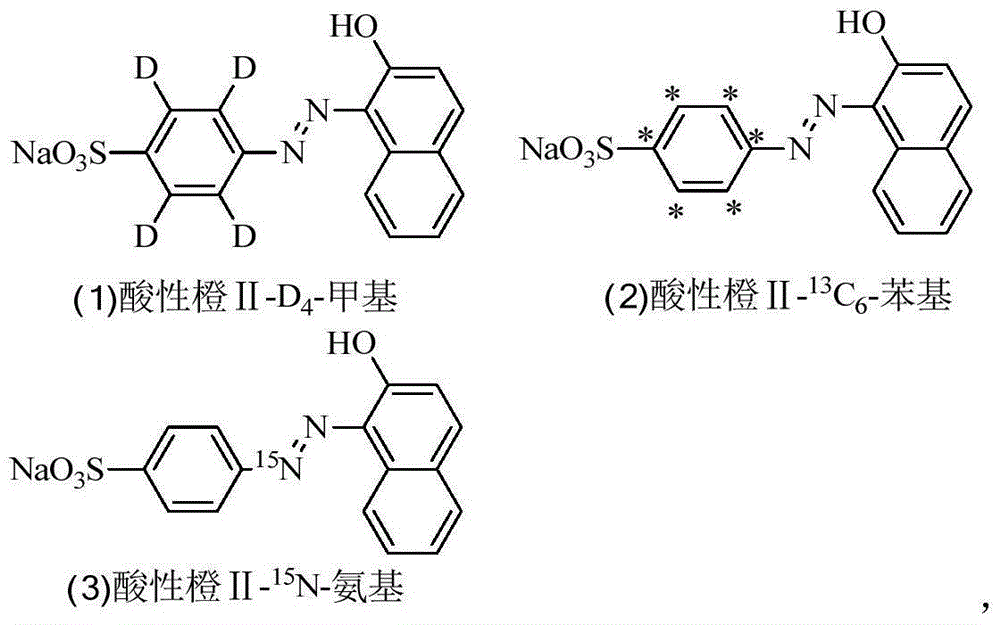
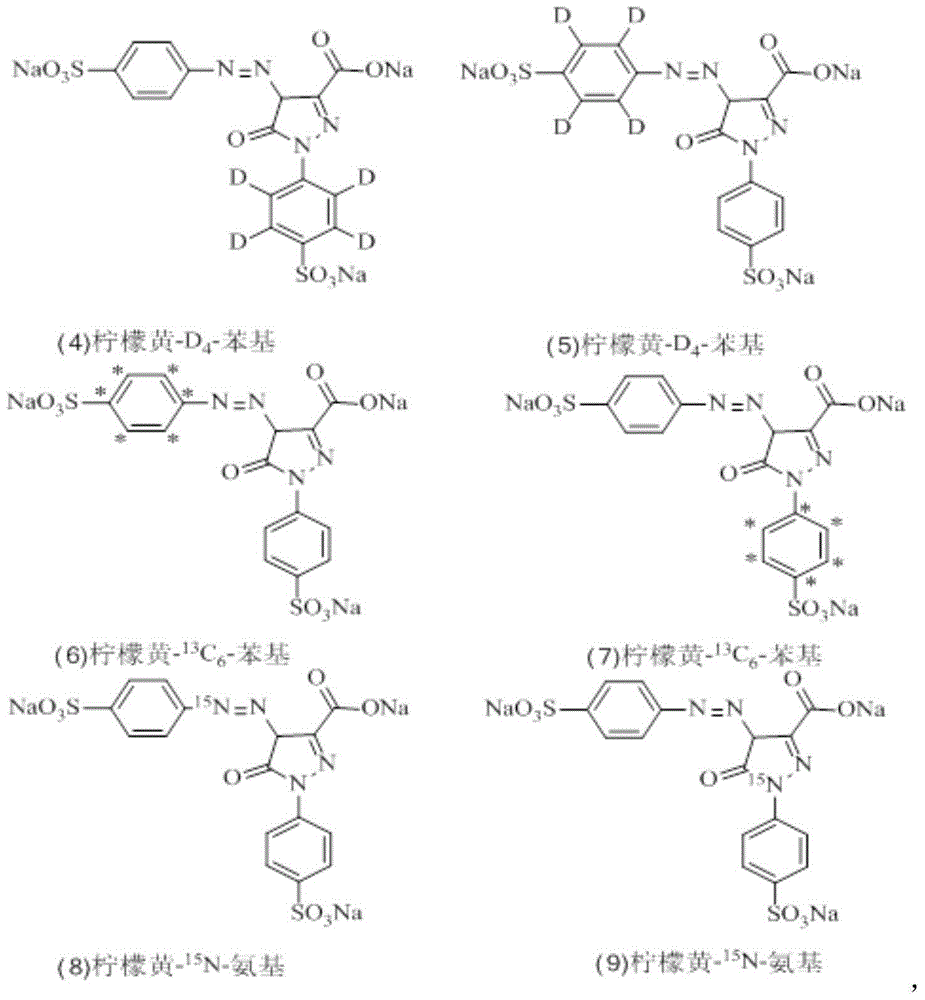
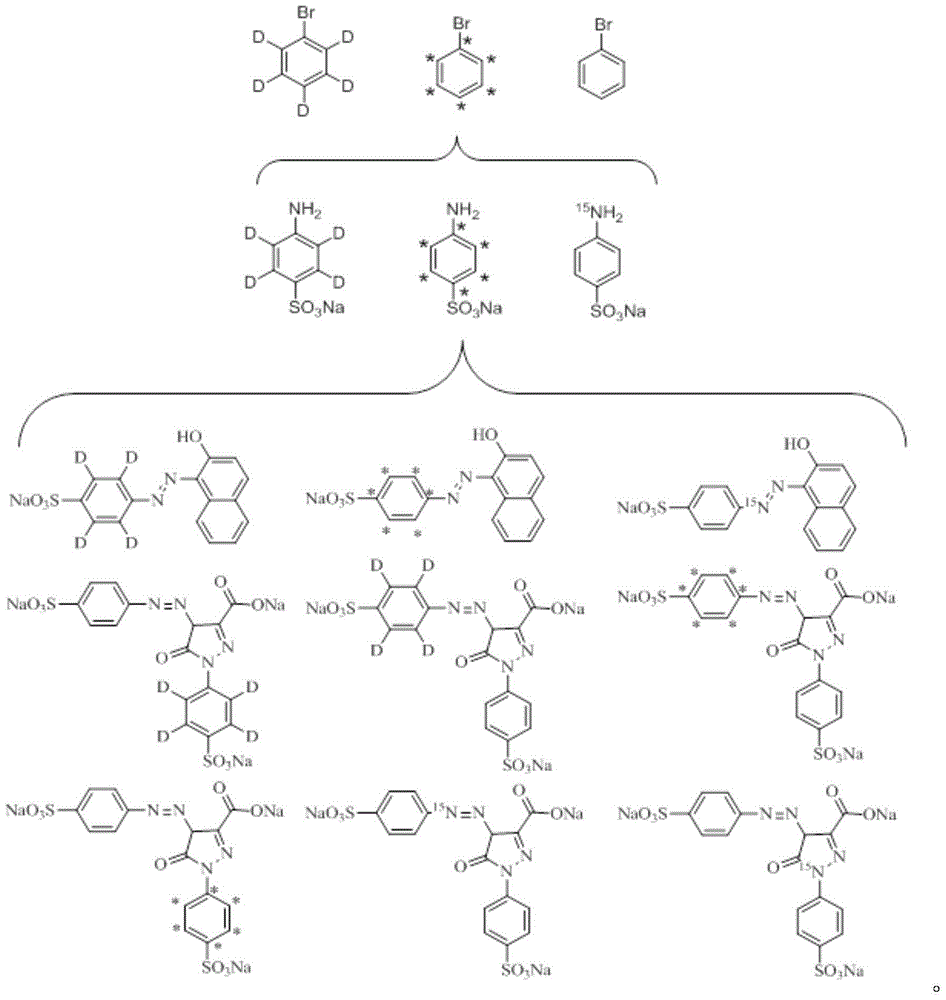
Synthesizing method for prohibited pigment labeled by stable isotope
A stable isotope and synthesis method technology, applied in the field of stable isotope-labeled prohibited pigment synthesis, can solve problems such as poor color, reddening when encountering alkali, fading when restored, endangering consumers' health, etc., and achieves good promotion prospects and good economy. And use value, the effect of simple process route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] A. Stable isotope labeled aniline-D 5 Synthesis
[0042] Add bromobenzene-D to the flask successively 5 , ammonia water, bromobenzene-D 5 The molar ratio with ammonia water is 1:10, respectively add ferric chloride and ferric oxide (the molar ratio of the two is 1:1), and distilled water with a reaction volume of 20%, stir, heat to 60°C, and react for 18h. After the reaction, extract, wash and dry. The crude yield is 68.5%, and the distillation yield is 42.0%. The HPLC detection purity is 99.4%, and the abundance is 99.2%.
[0043] B. Synthesis of Stable Isotope Labeled Sodium p-Sulfuran
[0044] Add stable isotope-labeled aniline-D to the flask 5 , then sequentially add oleum, aniline-D 5 The molar ratio of oleum to oleum is 1:2, then add ethylene glycol, aniline-D 5 The molar ratio with ethylene glycol is 1:10. The reaction was sealed in vacuum, the reaction temperature was controlled at 120° C., and the reaction was stopped after 4 hours. Let the reactant st...
Embodiment 2
[0048] A. Stable isotope labeled aniline-D 5 Synthesis
[0049] Add bromobenzene-D to the flask successively 5 , ammonia water, bromobenzene-D 5 The molar ratio with ammonia water is 1:15, respectively add ferric chloride and ferric oxide (the molar ratio of the two is 1:1), and distilled water with a reaction volume of 20%, stir, heat to 60°C, and react for 18h. After the reaction, extract, wash and dry. The crude yield is 68.5%, and the distillation yield is 42.0%. The HPLC detection purity is 99.3%, and the abundance is 99.1%.
[0050] B. Synthesis of Stable Isotope Labeled Sodium p-Sulfuran
[0051] Add stable isotope-labeled aniline-D to the flask 5 , then sequentially add oleum, aniline-D 5 The molar ratio with oleum is 1:2.5, then add o-dichlorobenzene, aniline-D 5 The molar ratio to o-dichlorobenzene is 1:8. The reaction was sealed in vacuum, the reaction temperature was controlled at 140° C., and the reaction was stopped after 5 hours. Let the reactant stand ...
Embodiment 3
[0055] A. Stable isotope labeled aniline- 13 C 6 Synthesis
[0056] Add bromobenzene- 13 C 6 , ammonia water, bromobenzene- 13 C 6 The molar ratio with ammonia water is 1:15, respectively add ferric chloride and ferric oxide (the molar ratio of both is 1:1.5), and distilled water with 20% reaction volume, stir, heat to 100°C, and react for 20h. After the reaction, extract, wash and dry. The crude yield was 70.5%. After distillation, the yield was 46.0%. The HPLC detection purity was 99.4%, and the abundance was 99.5%.
[0057] B. Stable isotope labeled sodium p-aminobenzenesulfonate- 13 C 6 Synthesis
[0058] Stable isotope-labeled aniline was added to the flask- 13 C 6 , then sequentially added oleum, aniline- 13 C 6 The molar ratio with oleum is 1:3, then add ethylene glycol, aniline- 13 C 6 The molar ratio with ethylene glycol is 1:12. The reaction was sealed in vacuum, the reaction temperature was controlled at 160° C., and the reaction was stopped after 4 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com