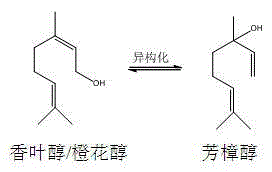
Method for continuously preparing linalool from allylic alcohol by isomerization process
A technology of allyl alcohol and isomerization, which is applied in the field of continuous preparation of linalool from allyl alcohol by isomerization method, can solve the problems of high waste rate, low reaction conversion rate and high reaction temperature, and achieves low price. , high yield and high selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Embodiment 1 A kind of method that allyl alcohol continuously prepares linalool through isomerization method
[0038] (1) Preparation of vanadium amine complex catalyst
[0039] Add 500.0g of xylene to a 1000ml three-necked flask equipped with a thermometer, condenser and mechanical stirring, add 5.0g (0.0274mol) of vanadium pentoxide and 5g (0.0494mol) of triethylamine, turn on the mechanical stirring to raise the temperature, at 140 ℃ for 1 hour, lower the temperature, and then filter through a sand core funnel, and the filter cake is washed twice with ether to obtain a vanadium amine complex catalyst, about 5.9 g.
[0040] (2) Add reactants and catalysts
[0041] In the 1000ml there-necked flask that is furnished with thermometer, 60cm rectifying column and magnetic force stirrer, add 500.0g geraniol content and be 35.6% geraniol / nerol mixture with geraniol content, add vanadium amine Complex catalyst 5.9g.
[0042] (3) Control of isomerization reaction conditions...
Embodiment 2
[0049] Embodiment 2 A kind of method that allyl alcohol continuously prepares linalool through isomerization
[0050] (1) Preparation of catalyst vanadium amine complexes
[0051]Add 500.0g of xylene, 20g (0.133mol) of vanadium trioxide and 8.5g (0.144mol) of n-propylamine into a 1000ml three-neck flask equipped with a thermometer, condenser and mechanical stirring, and turn on mechanical stirring to raise the temperature. Keep warm for 2 hours, lower the temperature, and then filter through a sand core funnel. The filter cake is washed twice with ether to obtain about 25.2 g of vanadium amine complex catalyst.
[0052] (2) Add reactants and catalysts
[0053] Add 500.0 g of geraniol with a content of 98.7% to a 1000 ml three-necked flask equipped with a thermometer, a 60 cm rectifying tower and a magnetic stirrer, and 25.2 g of a vanadium amine complex catalyst.
[0054] (3) Control of isomerization reaction conditions
[0055] Stir and heat up, turn on the vacuum pump to ...
Embodiment 3
[0061] Embodiment 3 A kind of method that allyl alcohol continuously prepares linalool through isomerization method
[0062] (1) Preparation of vanadium amine complex catalyst
[0063] Add 500.0g xylene, 30g (0.256mol) ammonium metavanadate and 10.5g (0.172mol) ethanolamine to a 1000ml three-neck flask equipped with a thermometer, condenser and mechanical stirring, turn on the mechanical stirring to raise the temperature, and keep it warm at 140°C After 2 hours, the temperature was lowered, and then filtered through a sand core funnel, and the filter cake was washed twice with ether to obtain about 33.6 g of vanadium amine complex catalyst.
[0064] (2) Add reactants and catalysts
[0065] In a 1000ml three-neck flask equipped with a thermometer, a 60cm rectifying tower and a magnetic stirrer, 500.0g of nerolidol with a content of 98.0% was added, and 33.6g of a vanadium amine complex catalyst was added.
[0066] (3) Control of isomerization reaction conditions
[0067] Sti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com