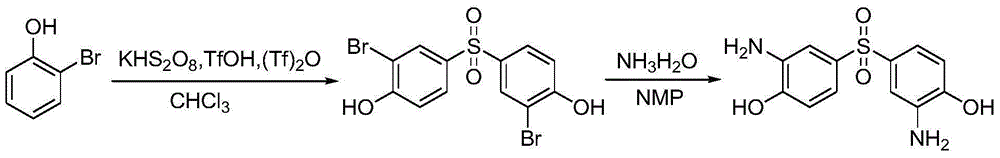
Preparation method of 3,3'-diamino-4,4'dyhydroxy diphenyl sulfone
A technology of dihydroxydiphenyl sulfone and diamino is applied in the field of preparation of monomer 3,3'-diamino-4,4'-dihydroxydiphenyl sulfone, and can solve long reaction steps, difficulty in filtration and difficulty in separation and other problems, to achieve the effect of solvent recovery and application, simple operation and good repeatability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Add 17.3 grams of o-bromophenol, 250 milliliters of chloroform, 84.6 grams of trifluoromethanesulfonic anhydride, 45 grams of trifluoromethanesulfonic acid and 20.2 grams of potassium hydrogen persulfate in the reaction flask (the molar ratio is: o-bromophenol: potassium hydrogen persulfate : Trifluoromethanesulfonic acid: Trifluoromethanesulfonic anhydride: Chloroform=1:1.2:3:3:31), heated up to 50°C for reaction under stirring, after the reaction of the raw materials was complete, the solvent was distilled off under reduced pressure, and the solid was precipitated by cooling down. After filtration, the filter cake was washed with aqueous sodium acetate, washed with water and dried to obtain 18.5 g of 3,3'-dibromo-4,4'-dihydroxydiphenylsulfone, with a yield of 90.5%.
Embodiment 2
[0021] Add 17.3 grams of o-bromophenol, 224 milliliters of chloroform, 56.4 grams of trifluoromethanesulfonic anhydride, 37.5 grams of trifluoromethanesulfonic acid and 18.5 grams of potassium hydrogen persulfate in the reaction flask (the molar ratio is: o-bromophenol: potassium hydrogen persulfate : trifluoromethanesulfonic acid: trifluoromethanesulfonic anhydride: chloroform=1:1.1:2.5:2:28), heated up to 40°C for reaction under stirring, after the reaction of the raw materials was complete, the solvent was evaporated under reduced pressure, and the solid was precipitated by cooling down. After filtration, the filter cake was washed with aqueous sodium acetate, washed with water and dried to obtain 16.4 g of 3,3'-dibromo-4,4'-dihydroxydiphenylsulfone, with a yield of 80.35%.
Embodiment 3
[0023] Add 17.3 grams of o-bromophenol, 272 milliliters of chloroform, 112.8 grams of trifluoromethanesulfonic anhydride, 52.5 grams of trifluoromethanesulfonic acid and 21.9 grams of potassium hydrogen persulfate in the reaction flask (the molar ratio is: o-bromophenol: potassium hydrogen persulfate : trifluoromethanesulfonic acid: trifluoromethanesulfonic anhydride: chloroform=1:1.3:3.5:4:32), heated up to 60°C for reaction under stirring, after the reaction of the raw materials was complete, the solvent was evaporated under reduced pressure, and the solid was precipitated by cooling down. After filtration, the filter cake was washed with aqueous sodium acetate, washed with water and dried to obtain 18.45 g of 3,3'-dibromo-4,4'-dihydroxydiphenylsulfone, with a yield of 90.47%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com