The synthetic method of kainic acid
A synthesis method and technology of kainic acid are applied in the field of synthesis of natural product kainic acid, and achieve the effects of simple operation process, low price and short reaction steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
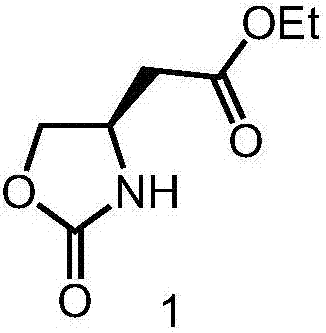
[0020] 1. Add 1.37g (59.6mmol) sodium metal into 120mL dry absolute ethanol, stir at room temperature for 2 hours to completely dissolve the sodium metal, and obtain a 0.5mol / L sodium ethoxide solution. Add 5.6g (23.8mmol) (S)-5-oxo-tetrahydrofuran-3-carbamate benzyl ester into the flask, stir at room temperature for 3 hours, add 60mL 1mol / L hydrochloric acid aqueous solution to quench the reaction, and use 100mL ethyl acetate Extracted 3 times, combined the organic phases, dried with anhydrous sodium sulfate, filtered, concentrated by rotary evaporator, and purified by column chromatography (the eluent was a mixture of petroleum ether and ethyl acetate with a volume ratio of 3:1) , to obtain 4.0 g of white solid compound 1 with a yield of 97%.
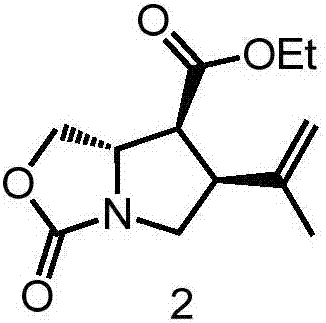
[0021] 2. Add 277mg (6.93mmol) of sodium hydride and 200mL of dry N,N-dimethylformamide into the reaction flask, and cool to -40°C under the protection of argon; dissolve 1.0g (5.77mmol) of compound 1 In 50mL of dry N,N-dimethylforma...
Embodiment 2
[0027] In step 2 of Example 1, 15 mg (0.3753 mmol) of sodium hydride and 2 mL of dry N,N-dimethylformamide were added to the reaction flask, and cooled to -40°C under the protection of argon; 50 mg ( 0.2887mmol) of compound 1 was dissolved in 1mL of dry N,N-dimethylformamide, slowly injected into the reaction vial with a syringe, and stirred at -40°C for 30 minutes; 58.2mg (0.3176mmol) of (E) -4-Bromo-1-chloro-2-methyl-2-butene was dissolved in 1 mL of dry N,N-dimethylformamide, injected slowly into the reaction vial with a syringe, and continued at -40 °C Stir for 30 minutes; warm the reaction to room temperature, add 480 mg (2.887 mmol) of dry potassium iodide and 0.3 mL (0.433 mmol) of lithium bistrimethylsilylamide, stir for 3 hours, and quench with saturated aqueous ammonium chloride solution after the reaction is complete reaction, extracted 3 times with 10mL ethyl acetate, combined the organic phases, successively washed with saturated sodium chloride (3 × 10mL), dried ...
Embodiment 3
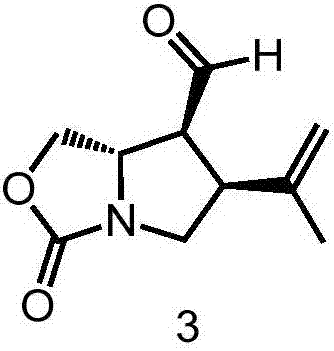
[0029] In step 3 of Example 1, the 1.52g (4.04mmol) of pyridinium dichromate used was replaced with 1.33g (3.14mmol) of Dess-Martin oxidant, and the other steps of this step were the same as in Example 1 to obtain 308mg of colorless Transparent liquid compound 3, its yield is 85%. Other steps were the same as in Example 1 to obtain kainic acid with a total yield of 16.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com