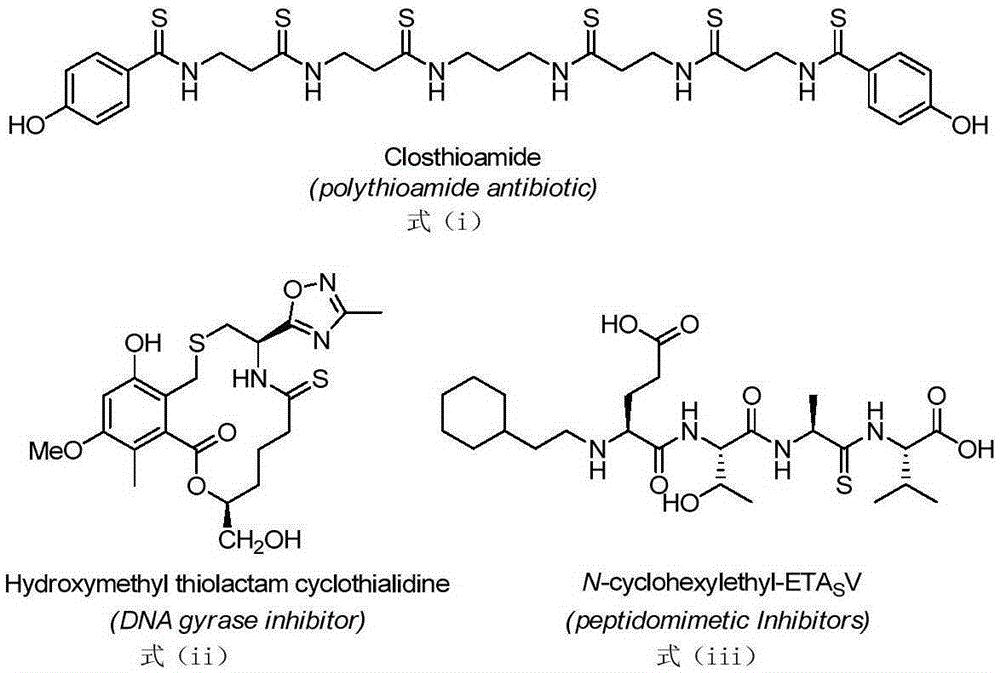
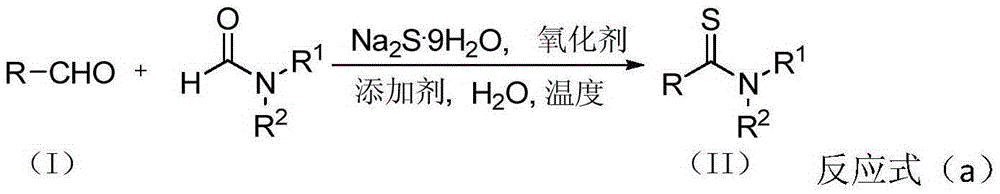
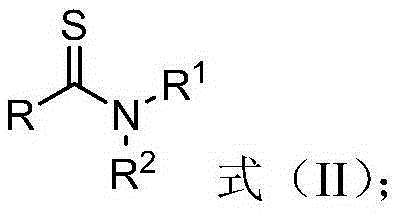
Thioamide compound and compounding method thereof
A technology of thioamides and synthetic methods, applied in the fields of steroids and organic chemistry, which can solve the problems of harsh substrate reaction conditions, environmental and human harm, and limited substrate universality, and achieve stereospecificity The effect of maintenance, wide substrate universality and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Synthesis of compound 2a:
[0057]
[0058] Add Na to the reaction tube 2 S·9H 2 O (1.05mmol, 252mg), isovaleraldehyde (0.3mmol, 25.8mg), N-formylpiperidine (1.5mmol, 169.5mg), pyridine (1.5mmol, 118.5mg) dissolved in water (0.6mL) was added reaction tube, and then K 2 S 2 o 8 (0.54mmol, 146.0mg) was added to the reaction system and stirred at 100°C for 8 hours. After the reaction was detected by TLC, it was lowered to room temperature, 10mL of water was added to the system for dilution, and then ethyl acetate (10mL*3) was added for extraction. Dry over anhydrous sodium sulfate, filter, concentrate, and separate by column chromatography to obtain yellow liquid 2a (33.8 mg, 61%), R f =0.55(PE:EA=10:1); 1 HNMR (400MHz, CDCl 3 )δ4.38-4.25(m,2H),3.76-3.68(m,2H),2.80(d,J=7.3Hz,2H),2.24-2.07(m,1H),1.78-1.60(m,6H) ,1.00(d,J=6.7Hz,6H) 13 CNMR (100MHz, CDCl 3 )δ201.4,52.0,51.6,51.1,28.9,26.9,25.4,24.1,22.4; IR (film)2937,2361,1487,1440,1265,1149,1079,1002,852cm -1 ...
Embodiment 2
[0060] Synthesis of compound 2b:
[0061]
[0062] Add Na to the reaction tube 2 S·9H 2 O (1.05mmol, 252mg), isovaleraldehyde (0.3mmol, 25.8mg), N-formylmorpholine (1.5mmol, 172.5mg), pyridine (1.5mmol, 118.5mg) dissolved in water (0.6mL) was added reaction tube, and then K 2 S 2 o 8 (0.54mmol, 146.0mg) was added to the reaction system, stirred at 100°C for 9 hours, after the reaction was detected by TLC, it was lowered to room temperature, 10mL of water was added to the system for dilution, and ethyl acetate (10mL*3) was added for extraction. Dry over anhydrous sodium sulfate, filter, concentrate, and separate by column chromatography to obtain light yellow liquid 2b (32.0mg, 57%), R f =0.6(PE:EA=5:1); 1 HNMR (400MHz, CDCl 3 )δ4.44-4.25(m,2H),3.86-3.62(m,6H),2.76(d,J=7.3Hz,2H),2.17-2.06(m,1H),0.99(d,J=6.7Hz ,6H); 13 CNMR (100MHz, CDCl 3 )δ203.2,66.6,66.5,51.6,50.4,50.1,29.1,22.3; IR (film)2961,2925,2866,1478,1431,1259,1115,1021,797cm -1 ; MS(EI) m / z(%) 187(100),...
Embodiment 3
[0064] Synthesis of compound 2c:
[0065]
[0066] Add Na to the reaction tube 2 S·9H 2 O (1.05mmol, 252mg), 3-phenylpropanal (0.3mmol, 40.2mg), N, N-dimethylformamide (1.5mmol, 109.5mg), pyridine (1.5mmol, 118.5mg) were dissolved in water (0.6mL) into the reaction tube, and then K 2 S 2 o 8 (0.54mmol, 146.0mg) was added to the reaction system and stirred at 100°C for 8 hours. After the reaction was detected by TLC, it was lowered to room temperature, 10mL of water was added to the system for dilution, and then ethyl acetate (10mL*3) was added for extraction. Dry over anhydrous sodium sulfate, filter, concentrate, and separate by column chromatography to obtain orange liquid 2c (48.0mg, 83%), R f =0.4(PE:EA=5:1); 1 HNMR (400MHz, CDCl 3 )δ7.32-7.27(m,2H),7.24-7.20(m,3H),3.48(s,3H),3.14(s,3H),3.13-3.09(m,2H),3.09-3.03(m, 2H); 13 CNMR (100MHz, CDCl 3 )δ203.1, 140.6, 128.5, 128.4, 126.4, 44.9, 44.6, 41.5, 35.7; -1 ; MS (EI) m / z (%) 193 (100), 88 (90).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com