Preparation method for human antithrombin III
An antithrombin, gel technology, applied in the direction of protease inhibitors, animal/human peptides, peptide sources, etc., can solve the problem of low AT-III production qualification rate, lack of targeted treatment measures, lack of removal measures, etc. problems, to achieve the effect of improving the safety of clinical use, reducing the probability of protein activation, and reducing the load
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
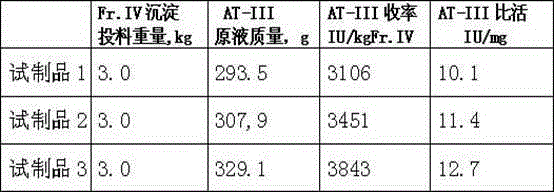
Embodiment 1
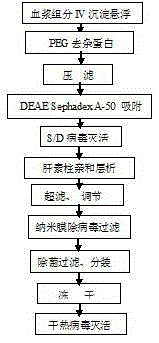
[0043] 1. Put 3kg of component IV precipitate into 30kg buffer solution, the buffer solution composition is 0.02M HCL-TRIS, 0.15M sodium chloride, pH7.50-7.60, the temperature is controlled at 10°C, and stirred for 6 hours to fully dissolve ;
[0044] 2. Add polyethylene glycol to the above suspension to 6%, stir for 0.5 hours, then filter with a Supradur50P filter plate produced by Pall in series with a 0.45 μm filter element, collect the clarified filtrate, and pre-wash the filter plate with dissolution buffer before filtering and filter elements;
[0045] 3. Add pre-swelled and balanced DEAESephadexA-50 gel to the above filtrate, add 33g according to the dry gel, the buffer solution is composed of 0.02MTRIS-HCL, 0.15M sodium chloride, pH6.50-6.50; slowly stir for 1.5 hours, Stand still for 5 minutes; Then filter and collect the filtrate;
[0046] 4. Add Tween80 to 1.0% (wt%) and TNBP (tributyl phosphate) to 0.3% (wt%) to the above filtrate, stir well, heat up to 24-26°C, ke...
Embodiment 2
[0055] 1. Put 3kg of component IV precipitate into 60kg of buffer solution, the buffer solution is composed of 0.01M HCL-TRIS, 0.15M sodium chloride, pH8.50-8.60, the temperature is controlled at 20°C, and stirred for 4 hours to fully dissolve it ;
[0056] 2. Add polyethylene glycol to the above suspension to 3%, stir for 1.5 hours, then filter with a Supradur50P filter plate produced by Pall in series with a 0.45 μm filter element, collect the clarified filtrate, and pre-wash the filter plate with dissolution buffer before filtering and filter elements;
[0057] 3. Add pre-swelled and balanced DEAESephadexA-50 gel to the above filtrate, add 33g according to the dry gel, the buffer solution is composed of 0.01MTRIS-HCL, 0.15M sodium chloride, pH6.90-7.10; slowly stir for 1 hour, After standing for 10 minutes; then filter and collect the filtrate;
[0058] 4, with embodiment one;
[0059] 5. Put the above filtrate on the HeparinSepharose6FF chromatography column. The column...
Embodiment 3
[0064] 1. Put 3kg of component IV precipitate into 45kg buffer solution, the buffer solution composition is 0.02M HCL-TRIS, 0.1M sodium chloride, pH9.40-9.50, the temperature is controlled at 25°C, and stirred for 2.5 hours to fully dissolve it ;
[0065] 2. Add polyethylene glycol to the above suspension to 4.5%, stir for 1 hour, then filter with a Supradur50P filter plate produced by Pall in series with a 0.45 μm filter element, collect the clarified filtrate, and pre-wash the filter plate with dissolution buffer before filtering and filter elements;
[0066] 3. Add pre-swelled and balanced DEAESephadexA-50 gel to the above filtrate, add 36g according to the dry gel, the buffer solution is composed of 0.02MTRIS-HCL, 0.1M sodium chloride, pH7.40-7.50; slowly stir for 0.5 hours, Stand still for 15 minutes; Then filter and collect the filtrate;
[0067] 4, with embodiment one;
[0068] 5. Put the above filtrate on the HeparinSepharose CL-6B chromatography column. The column ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com