Preparation of glutamic acid modified polyethylene glycol monostearate and application of glutamic acid modified polyethylene glycol monostearate in target drug transfer
A technology of polyethylene glycol monostearate and monostearate, applied to the application of targeted materials in active targeted drug delivery systems, glutamic acid-polyethylene glycol monostearate In the field of preparation, it can solve the problems of ignoring the characteristics and advantages of transporters, and achieve good tumor targeting, easy preparation, and good tumor inhibitory effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
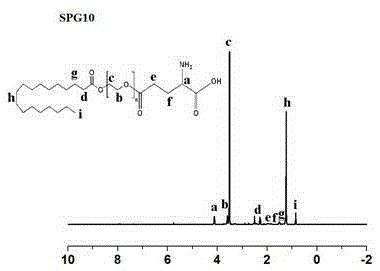
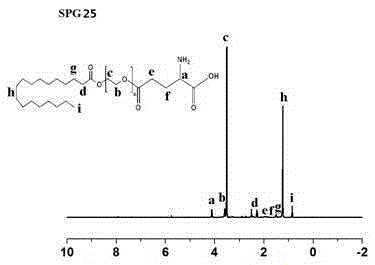
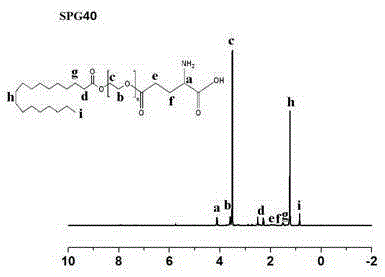
[0061] Preparation of glutamic acid-polyethylene glycol monostearate with four different PEG chain lengths.
[0062] N-benzyloxycarbonyl-L-glutamic acid-1-benzyl ester (Z-Glu-OBzl, II), dissolved in an appropriate amount of organic solvents such as dichloromethane and dimethyl sulfoxide, under the action of a catalyst, Such as: 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC) and 4-dimethylaminopyridine (DMAP), keep away from light in ice bath for 1h-2h, and then mix with Different chain length polyethylene glycol monostearate (SP n , Ⅰ) reacted under the protection of N2 at 30°C for 12h-48h, and obtained light yellow solid Ⅲ through separation and purification. Compound Ⅲ undergoes palladium-carbon reduction reaction, removes the protecting group of glutamic acid, and then obtains the final compound-glutamic acid-polyethylene glycol monostearate (SPG) through further separation and purification. n ,Ⅳ). The reaction formula is as follows:
[0063]
[00...
Embodiment 2
[0067] Glutamic acid-polyethylene glycol monostearate modified nanoparticles loaded with paclitaxel or coumarin 6 prepared by emulsification solvent evaporation method
[0068] Weigh 1 mg of paclitaxel or coumarin 6, dissolve in 1 mL of dichloromethane, add 1 mg or 2 mg of glutamic acid-modified polyethylene glycol monostearate or polyethylene glycol with different PEG chain lengths prepared in Example 1 alcohol monostearate, and 20 mg of polylactic polyglycolic acid copolymer. Add it to 5 mL of 1% polyvinyl alcohol aqueous solution, sonicate the probe at 300 W for 10 min, stir overnight, and centrifuge at 3500 rpm for 10 min to remove uncoated drugs.
[0069] The nanoparticles prepared in Example 2 are measured by dynamic light scattering and transmission electron microscopy for particle size and shape of micelles, the results are as follows: Figure 4 . Dynamic light scattering was used to measure glutamic acid-polyethylene glycol monostearate modified nanoparticles with d...
Embodiment 3
[0073] Stability Test of Glutamic Acid-Polyethylene Glycol Monostearate Modified Drug-loaded Nanoparticles in Plasma
[0074] Prepare the drug-loaded nanoparticles modified by glutamic acid-polyethylene glycol monostearate according to Example 2, place the nanoparticles in pH7.4 phosphate buffer containing 10% FBS, and measure the concentration of each nanometer by dynamic light scattering. Particle size at 0h, 1h, 2h, 4h, 6h8h, 10h, 12h and 24h.
[0075] Figure 5 The results showed that the glutamic acid-polyethylene glycol monostearate modified drug-loaded nanoparticles with different ligand modification densities and PEG chain lengths had better plasma stability.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com