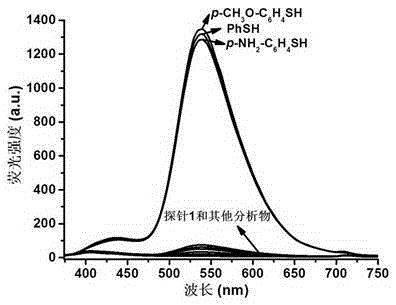
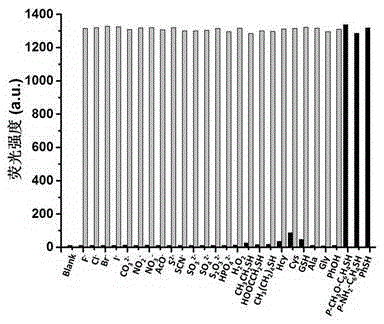
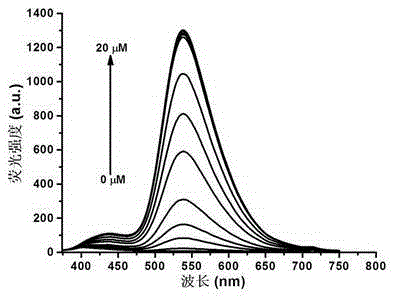
Benzoxazinone thiophenol fluorescent probe and preparation method thereof
A benzoxazinone and fluorescent probe technology, which is applied in the field of analysis and detection, can solve the problems of limiting practical application and long time required for detection, and achieves the effects of good fluorescence performance, low detection limit, and easy separation and purification.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Synthesis of Fluorescent Probe Compounds Based on Benzoxazinones and Thiophenols
[0029] In a 50mL single-necked flask, 7-hydroxy-3-methylbenzoxazinone (90mg, 0.6mmol), 2,4-dinitrobenzenesulfonyl chloride (149mg, 0.6mmol) and triethylamine ( 0.25mL, 1.8mmol) was dissolved in 5mL of dry tetrahydrofuran, stirred at room temperature for 2h, the solvent was distilled off under reduced pressure, the resulting residue was dissolved in dichloromethane, washed three times with 50mL of water, and the organic phase was dried with anhydrous sodium sulfate. The solvent was distilled off under reduced pressure to obtain a crude product, which was separated through a silica gel (200-300 mesh) column with dichloromethane / ethyl acetate=3 / 1 to obtain a yellow solid with a yield of 71%.
[0030] Wherein, 7-hydroxyl-3-methylbenzoxazinone can be according to literature D.R.Shridhar, B.Lal, N.K.Vaidya, K.K.Bhopale and H.N.Tripathi, Synthesis & biological activity of some 3-[2-(heteroaryl)-...
Embodiment 2
[0033] Synthesis of Fluorescent Probe Compounds Based on Benzoxazinones and Thiophenols
[0034] In a 50mL single-necked flask, 7-hydroxy-3-methylbenzoxazinone (90mg, 0.6mmol), 2,4-dinitrobenzenesulfonyl chloride (149mg, 0.6mmol) and triethylamine ( 0.25mL, 1.8mmol) was dissolved in 5mL of dry tetrahydrofuran, stirred at room temperature for 6h, the solvent was distilled off under reduced pressure, the resulting residue was dissolved in 50mL of dichloromethane, then washed three times with 50mL of water, and the organic phase was dried with anhydrous sodium sulfate , The solvent was distilled off under reduced pressure to obtain a crude product, which was separated through a silica gel (200-300 mesh) column with dichloromethane / ethyl acetate=3 / 1 to obtain a yellow solid with a yield of 66%.
Embodiment 3
[0036] Synthesis of Fluorescent Probe Compounds Based on Benzoxazinones and Thiophenols
[0037] In a 50mL single-necked flask, 7-hydroxy-3-methylbenzoxazinone (90mg, 0.6mmol), 2,4-dinitrobenzenesulfonyl chloride (224mg, 0.9mmol) and triethylamine ( 0.25mL, 1.8mmol) was dissolved in 5mL of dry tetrahydrofuran, stirred at room temperature for 2h, the solvent was distilled off under reduced pressure, the residue was dissolved in 50mL of dichloromethane, washed three times with 50mL of water, and the organic phase was dried with anhydrous sodium sulfate , The solvent was distilled off under reduced pressure to obtain a crude product, which was separated through a silica gel (200-300 mesh) column with dichloromethane / ethyl acetate=3 / 1 to obtain a yellow solid with a yield of 60%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com