Hydrogel applicable to cell adhesion and preparation method of hydrogel
A hydrogel and cell technology, applied in medical science, prosthesis, etc., can solve the problems of poor biocompatibility of hydrogel, damage to cells and tissues, weak mechanical properties, etc., and achieve easy control of synthesis conditions and biological phase. The effect of good capacitance and low synthesis cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0073] The preparation method of hydrogel of the present invention specifically comprises the following steps:
[0074] The above-prepared Ac-IIISLGK-NH 2 It is mixed with L-glutamine at a molar ratio of 1:1 and synthesized under enzymatic reaction conditions. During the enzymatic reaction, Ac-IIISLGK-NH 2 The final concentration of the solution was 7.27 mM, the concentration of transglutaminase TG was 0.9 U / mL, and the reaction temperature was controlled at 37° C. for 24 hours to obtain a hydrogel.
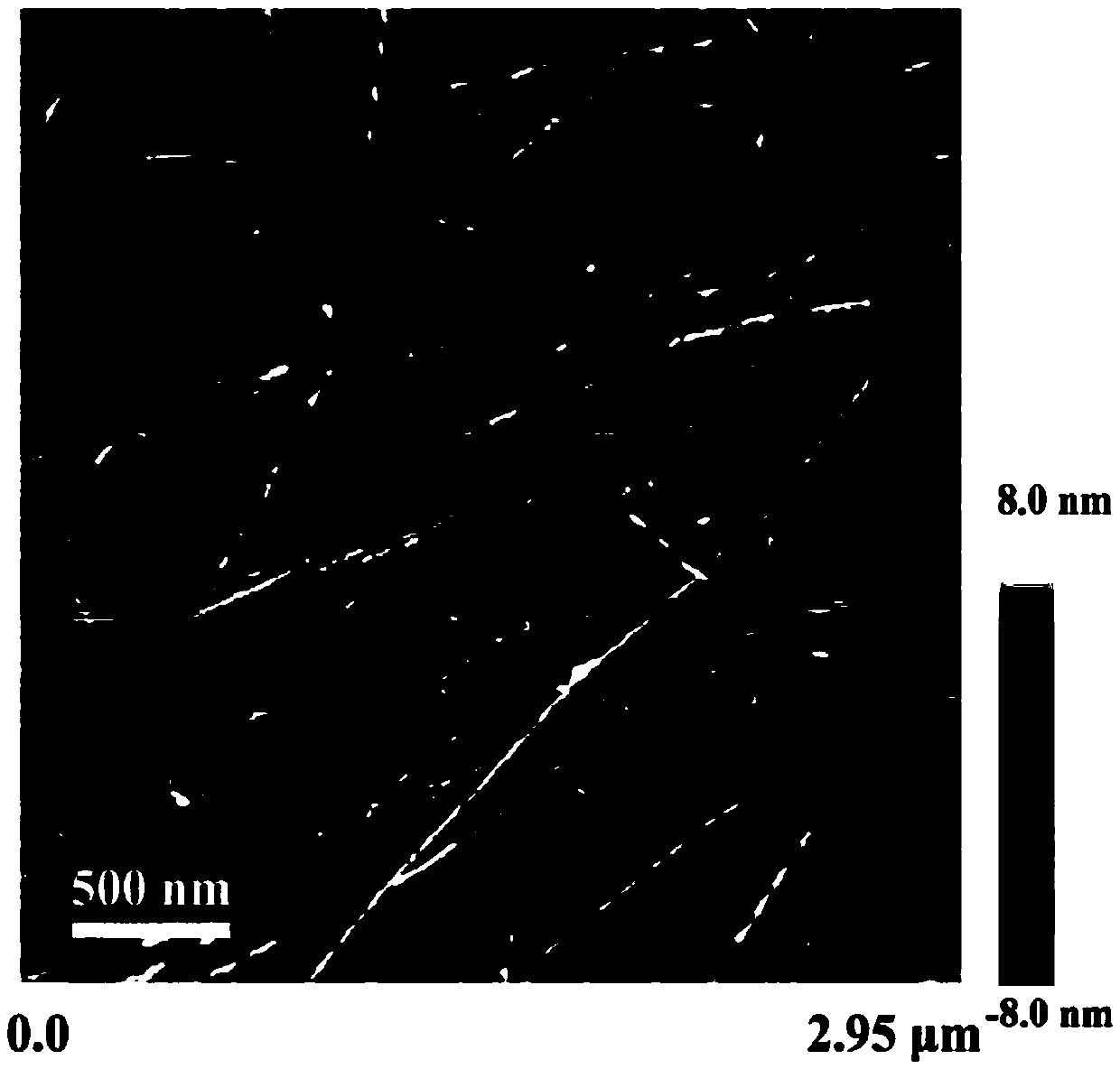
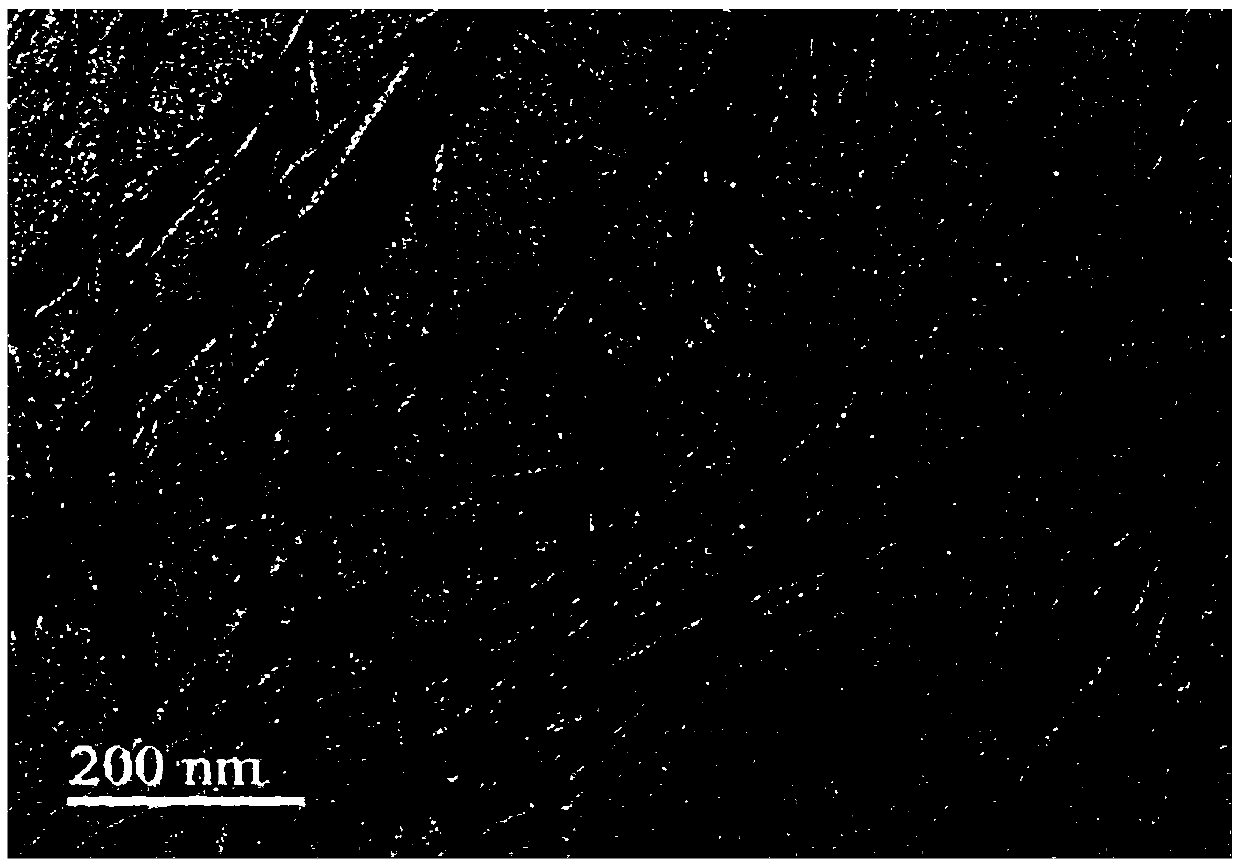
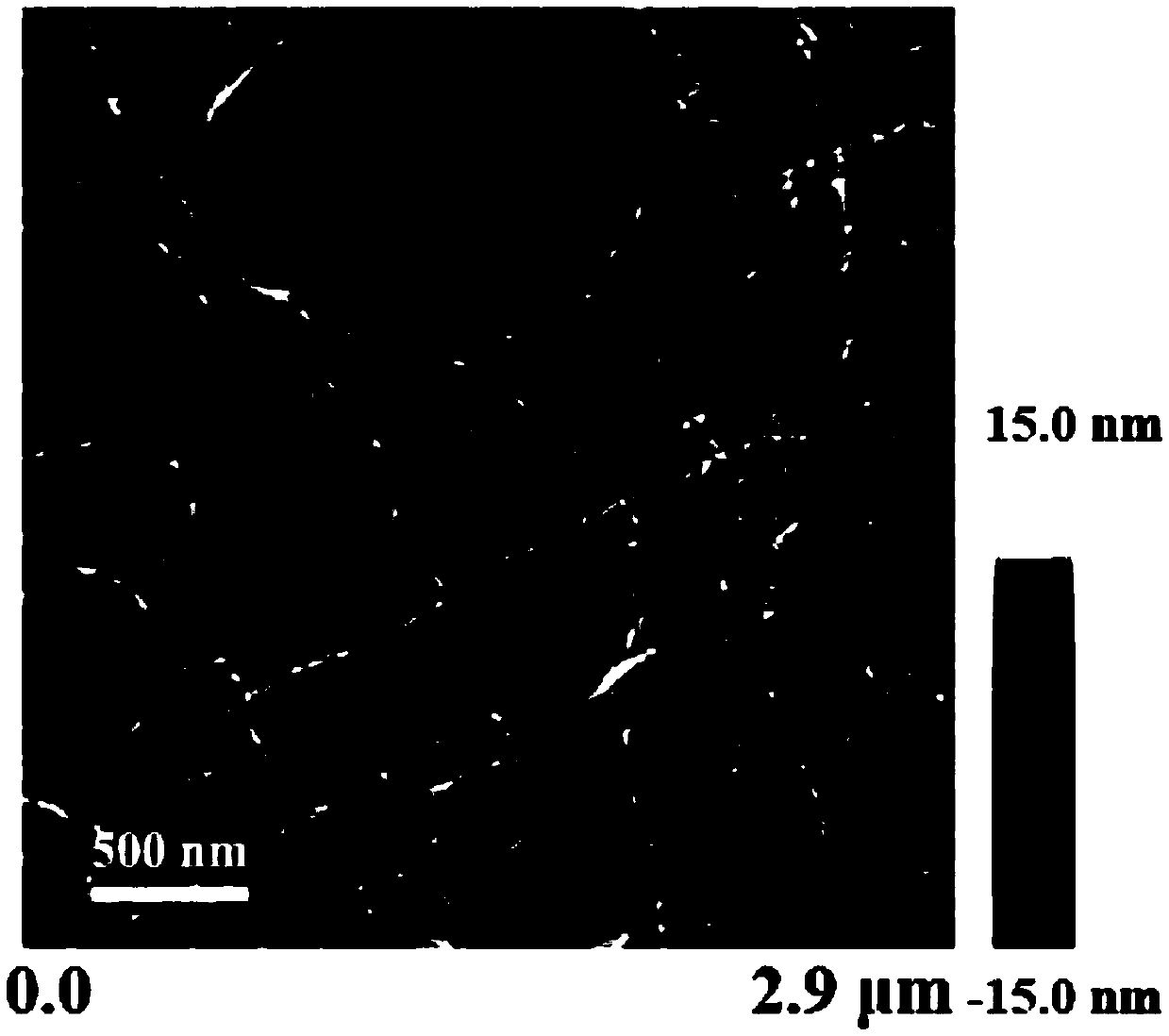
[0075] Detection: Take 10 μL of hydrogel sample dropwise on the surface of a new mica sheet, after static adsorption for 10s, blow dry the sample with high-purity nitrogen, AFM scanning, take a complete piece of hydrogel on the surface of the sealing film, place the carbon film A 400-mesh copper mesh was covered on the top of the gel and allowed to absorb for 1 min. After removing the copper mesh, use filter paper to absorb the residual liquid around the copper mesh. Stain with ...
Embodiment 2
[0077] The preparation method of hydrogel of the present invention specifically comprises the following steps:
[0078] 500 μL of Ac-IIISLGK-NH 2 (16mM) and L-Q (16mM) mixed solution at room temperature for 24 hours, add 25μL TG solution and the same amount of Ca 2+ The dependent solution, after being thoroughly mixed, was placed in a water bath at 37°C for 24 hours to obtain a hydrogel.
[0079] Mechanical strength detection of the hydrogel: the mechanical properties (viscoelasticity) of the gel formed by the hydrogel catalyzed by transglutaminase were characterized by a Haake rheometer, and the measuring module used was a cone with a diameter of 35 mm and a taper of 2°. The plate and the corresponding sample loading platform, the sample volume for each measurement is 500 μL, the temperature of the rheological experiment is 25 ° C, and after the sample reaction is catalyzed by transglutaminase for 24 hours, the stress scan is performed at a frequency of 1 Hz, and the scan ra...
Embodiment 3
[0088] The preparation method of hydrogel of the present invention specifically comprises the following steps:
[0089] The above-prepared Ac-IIISLGK-NH 2 It is mixed with L-glutamine at a molar ratio of 1:1 and synthesized under enzymatic reaction conditions. During the enzymatic reaction, Ac-IIISLGK-NH 2 The final concentration of the solution was 16 mM, the concentration of transglutaminase TG was 0.9 U / mL, and the reaction temperature was controlled at 38° C. for 36 hours to obtain a hydrogel.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com