Amine derivative containing adamantane structure and preparation method and application thereof
A technology for amine derivatives and adamantane, which is applied in the field of amine derivatives containing adamantane structure and their preparation, can solve the problems of less application research on adamantane and its derivatives, and achieves low raw material price and high yield. , the effect of a simple synthesis method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
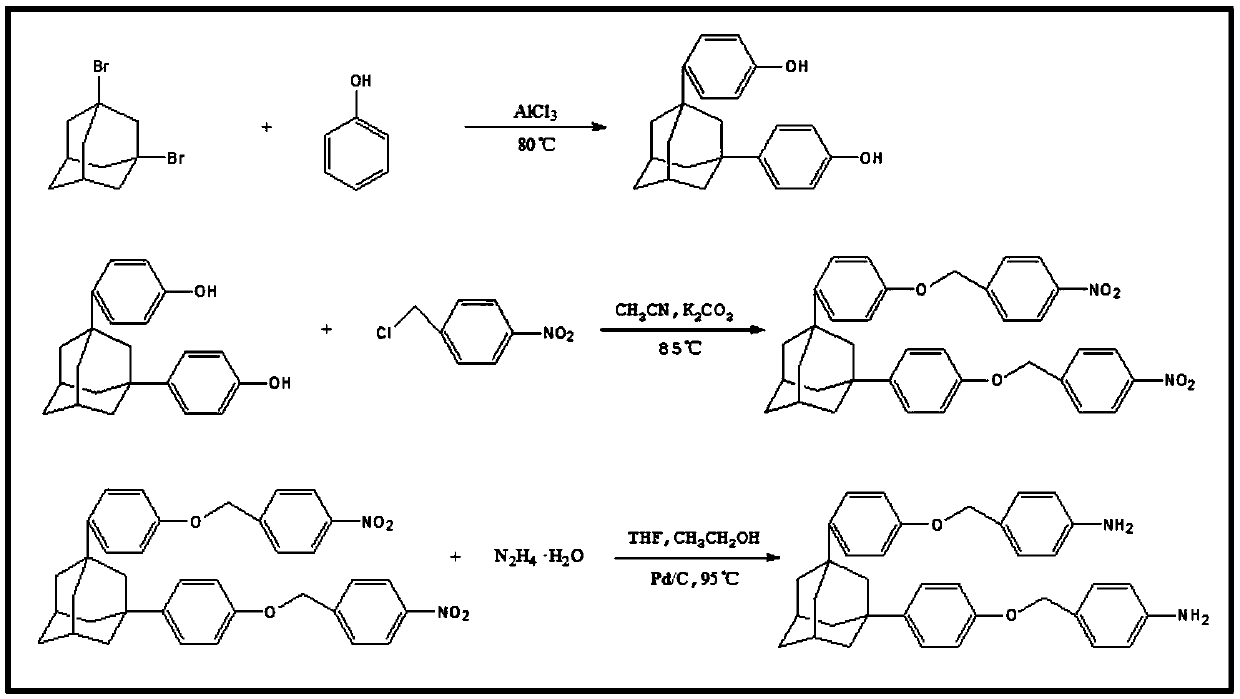
[0029] Step I:
[0030] In a clean 250ml three-neck round bottom flask, add a magnetic rotor of suitable size, 100g phenol and 1.2g anhydrous aluminum trichloride, connect the condensing reflux device and the tail gas absorption device (water or dilute sodium hydroxide solution) stably, and pass Inject nitrogen for 1 hour to prevent the oxidation of phenol during the reaction, then increase the temperature of the oil bath to 60°C. After the phenol is preheated and melted, add 15g of 1,3-dibromoadamantane into the flask and stir evenly, then raise the temperature of the oil bath to 80°C. Reaction 2h. Add 15g of 1,3-dibromoadamantane into the flask in 2 to 3 times, with an interval of 2.5h or 1.5h, and then continue the constant temperature reaction for 6h after adding, and finally pour it into hot water at 65°C and stir for washing. After suction filtration, repeat the previous operation on the filter residue until the color of the filtered filtrate is transparent, and dry the...
Embodiment 2
[0036] Step I is the same as in Example 1.
[0037] Step II:
[0038] Add the product of the first step (20g, 0.0625mol), p-nitrobenzyl chloride (25g, 0.146mol), potassium carbonate (25g, 0.181mol) and 200ml of acetonitrile into a 250ml one-necked flask, heat up to 75°C and stir under reflux for 8h. After the reaction was completed, suction filtered while it was hot, and the filter residue was stirred and washed twice with absolute ethanol and twice with 1mol / L dilute hydrochloric acid, and the target product was obtained after drying.
[0039] Step Ⅲ:
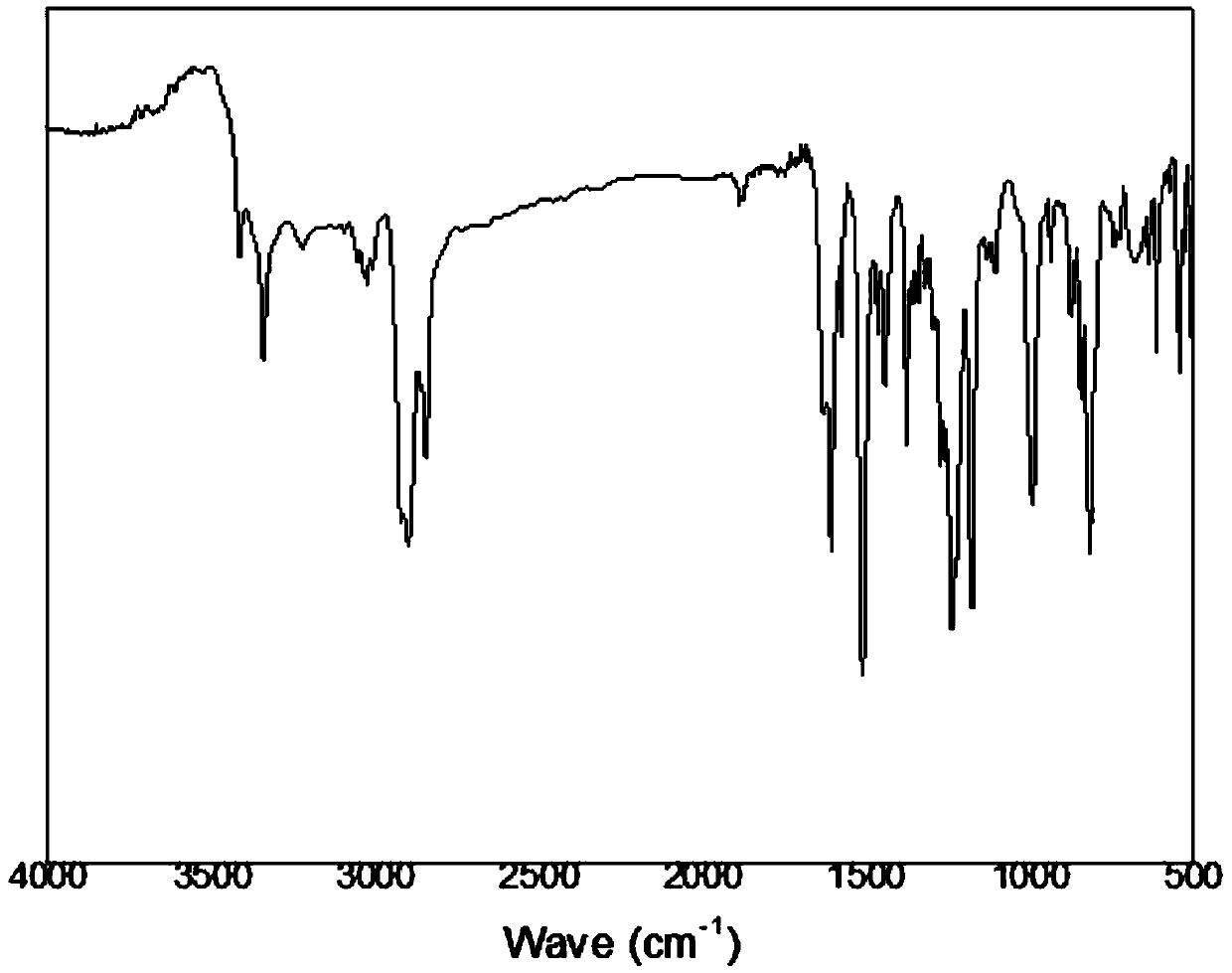
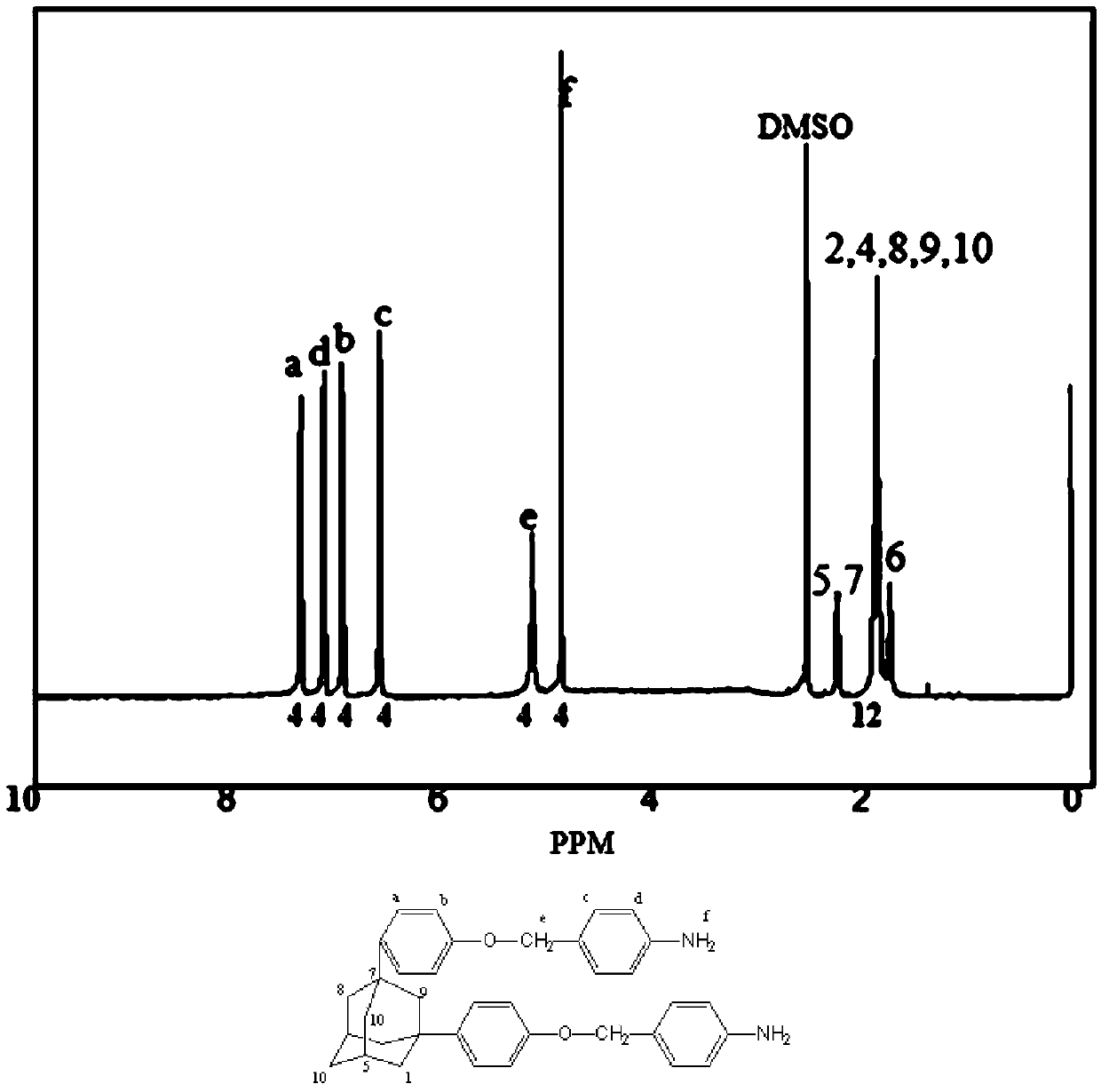
[0040] 20g of the second-step product, 0.5gPd / C (Pd10%), 300ml of tetrahydrofuran and 150ml of absolute ethanol were charged into a 1000ml three-neck round bottom flask and connected to a constant pressure dropping funnel and a condensing reflux tube. After nitrogen was introduced for 1 hour, the temperature rose to 95°C, and 100ml of hydrazine hydrate (85%) was put into a constant pressure dropping funnel and slowly added d...
Embodiment 3
[0042] Steps I and II are the same as in Example 1.
[0043] Step III is the same as in Example 2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com