Method for preparing doxepin hydrochloride using o-halogen methyl methyl benzoate as raw material
A technology for methyl ortho-halomethylbenzoate and doxepin hydrochloride, which is applied in the field of preparing doxepin hydrochloride and can solve problems such as low yield and low product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
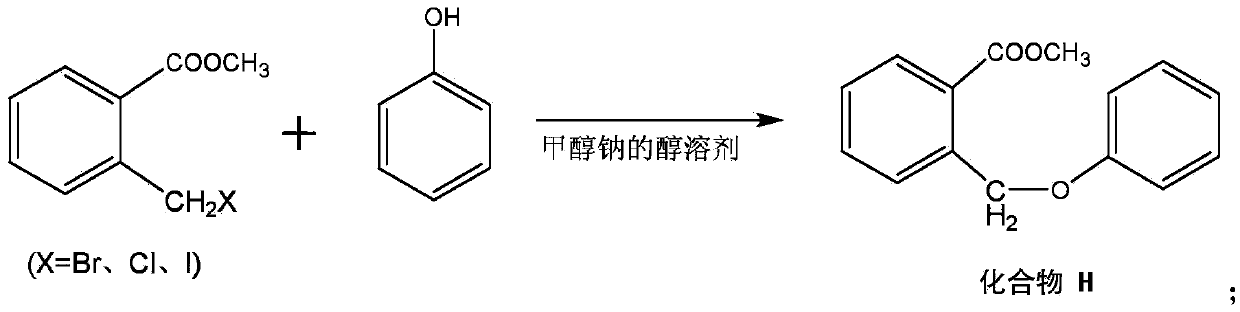
[0042] In the reaction vessel of 20L, put methyl o-bromomethylbenzoate, the ethanol solvent of sodium methylate that consumption is 3 (the total mass of methyl o-bromomethylbenzoate and phenol is 1 meter), adjust the temperature of reaction solution After reaching 50°C, start to drop phenol with a substance quantity of 1.05 (based on the substance quantity of methyl o-bromomethylbenzoate as 1), and the dropwise addition time is 1 h. After the dropwise addition was completed, after constant temperature reaction for 5 h, a known separation method was used to obtain methyl o-formate benzyl phenyl ether, which was named compound H.
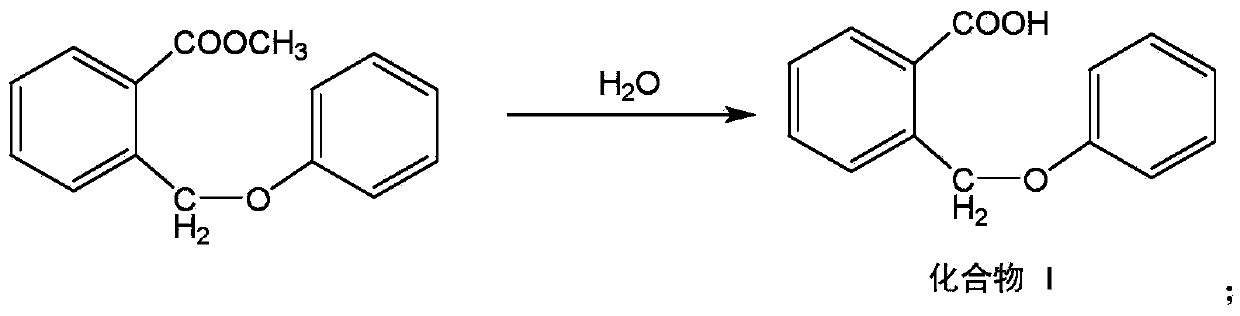
[0043] The above compound H and sodium hydroxide aqueous solution were placed in a reaction vessel, the temperature was adjusted at 30°C, the reaction was stirred for 8 hours, and (o-formyl)benzyl phenyl ether was separated by known separation means, which will be named as compound I.
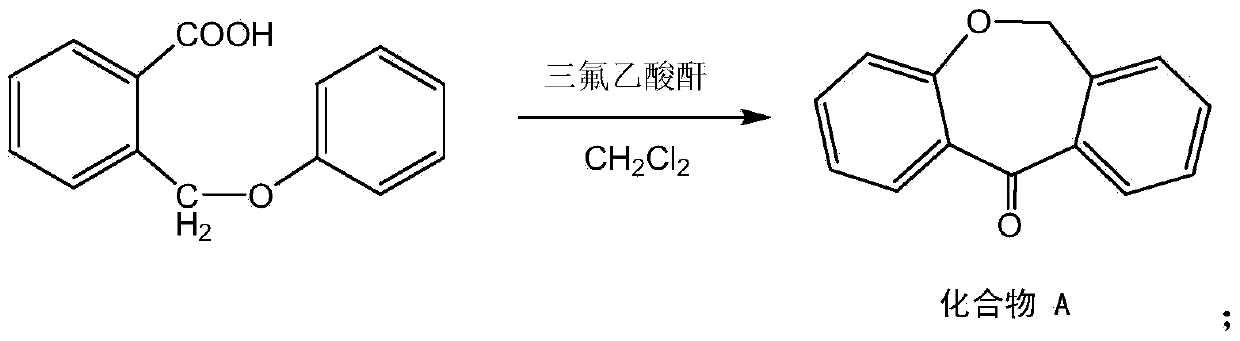
[0044] Put the above compound I, trifluoroacetic anhydride (based...
Embodiment 2
[0051] In the reaction vessel of 20L, put methyl o-bromomethylbenzoate, the ethanol solvent of sodium methylate that consumption is 10 (the total mass of methyl o-bromomethylbenzoate and phenol is 1 meter), adjust the temperature of reaction solution After reaching 60°C, start to drop phenol with a substance quantity of 1.15 (calculated that the substance quantity of methyl o-bromomethylbenzoate is 1), and the dropwise addition time is 1 h. After the dropwise addition was completed, after constant temperature reaction for 5 h, a known separation method was used to obtain methyl o-formate benzyl phenyl ether, which was named compound H.
[0052] Put the above-mentioned compound H and aqueous solution of sodium hydroxide into a reaction vessel, adjust the temperature at 40°C, stir the reaction for 4 hours, and obtain (o-formyl)benzylphenyl ether by using known separation methods, which will be named compound I.
[0053] Put the above compound I, trifluoroacetic anhydride (based ...
Embodiment 3
[0060] In the reaction vessel of 20L, put methyl o-chloromethylbenzoate, the ethanol solvent of sodium methylate that consumption is 6 (the total mass of methyl o-bromomethylbenzoate and phenol is 1 meter), adjust the temperature of reaction solution After reaching 55°C, phenol with a substance quantity of 1.10 (calculated as 1 substance quantity of methyl o-bromomethylbenzoate) was started to be added dropwise, and the dropwise addition time was 1 h. After the dropwise addition was completed, after constant temperature reaction for 3.5 h, a known separation method was used to obtain methyl o-formate benzyl phenyl ether, which was named compound H.
[0061] The above-mentioned compound H and sodium hydroxide aqueous solution were placed in a reaction vessel, the temperature was adjusted at 35°C, the reaction was stirred for 6 hours, and (o-formyl)benzyl phenyl ether was separated by known separation means, which will be named compound I.
[0062] Put the above compound I, trif...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com