Salt-tolerant esterase, coding gene of salt-tolerant esterase and application of salt-tolerant esterase
An esterase and gene technology, which is applied to salt-tolerant esterase and its encoding gene and application fields, can solve problems such as esterase gene cloning, and achieve the effect of good application value and good application potential.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1 Obtaining of esterase gene and verification of the function of synthesizing short-chain aromatic ester compounds
[0039] 1. Acquisition of esterase gene
[0040] From the environmental samples of traditional fermented food (soy sauce, fermented bean curd, soybean paste, etc.) in my country, a fermented food environmental metagenomic library was constructed. A medium containing 100 μg / ml ampicillin, 1% glycerol triphosphate, 1% glucose and 0.5% amino acid complex was used as a selection medium to screen esterase-positive clones from the library. Copy the cells in the library onto a solid selective medium plate with a replica inoculation needle, culture at 37°C for 3 days, and screen for positive clones with hydrolysis circles around the colonies.
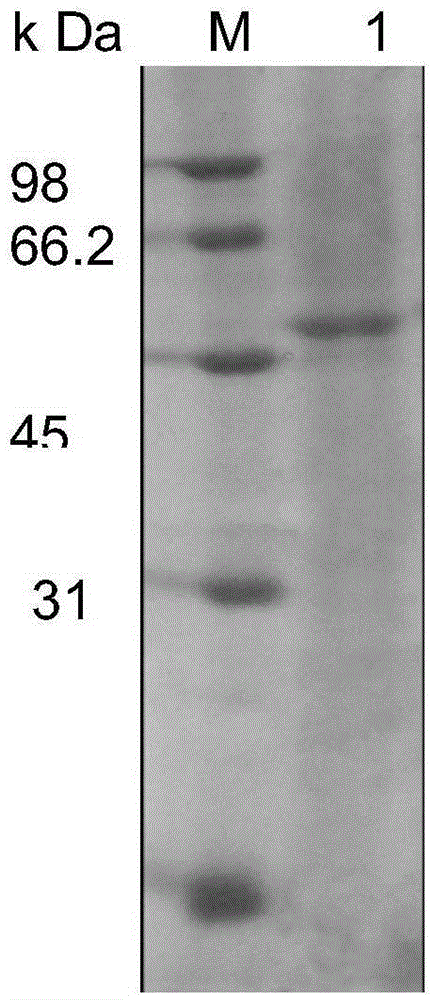
[0041] Extract the plasmid of the above-mentioned positive clone and send it to a sequencing company for sequencing to obtain a nucleic acid sequence of esterase, which consists of 948 bases, and its nucleic acid s...
Embodiment 2
[0053] Embodiment 2 Recombinant esterase EST_115 substrate specificity analysis
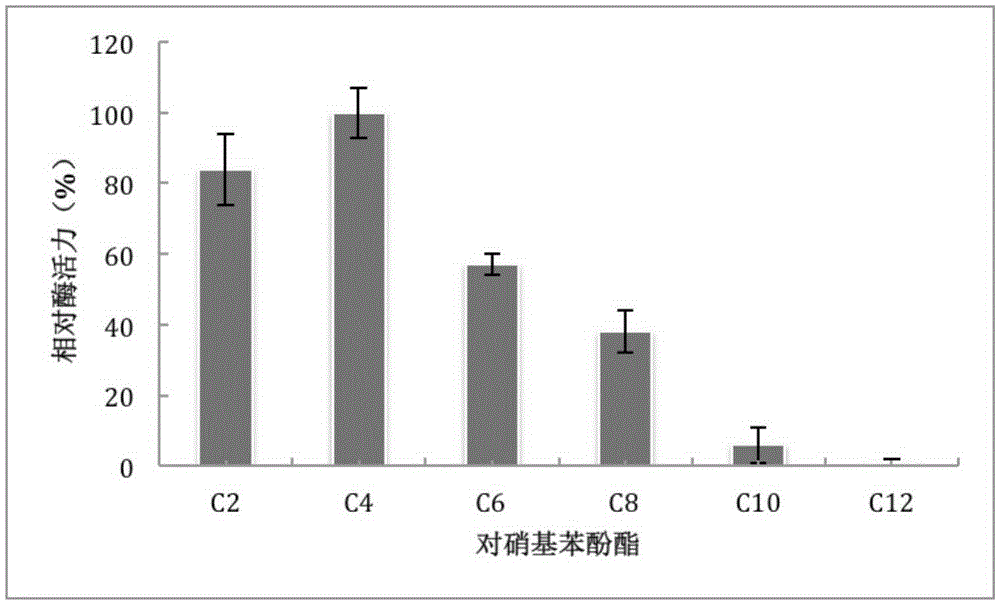
[0054] 100 mM Tris-HCl buffer (pH 7.0), 1 mM substrate, 10 μl of purified enzyme solution was added, and the absorbance value A405nm was measured at 35°C. Determination of substrates: p-nitrophenol acetate (C2), p-nitrophenol butyrate (C4), p-nitrophenol hexanoate (C6), p-nitrophenol octanoate (C8), p-nitrophenol octanoate (C8), p-nitrophenol Nitrophenol Caprate (C10), p-Nitrophenol Dodecanoate (C12). The result is as figure 1 As shown, the recombinant esterase EST_115 has catalytic activity on p-nitrophenol esters with shorter acyl carbon chains (C2, C4, C6 and C8), and the substrate with the highest catalytic activity is p-nitrophenol butyrate (C4) , followed by p-nitrophenol acetate (C2) and p-nitrophenol hexanoate (C6).
Embodiment 3
[0055] Example 3 Recombinant Esterase EST_115 Salt Tolerance Analysis
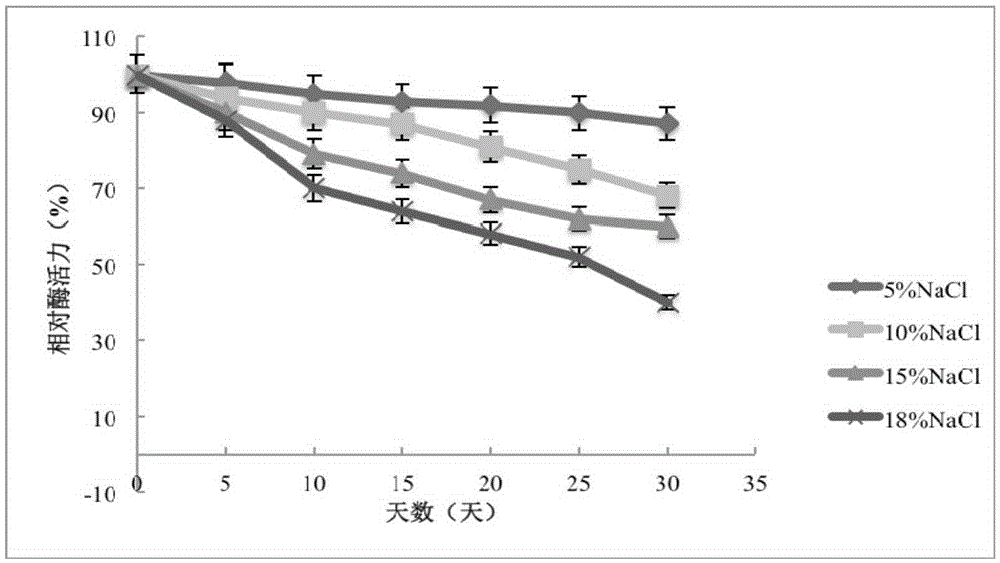
[0056] The purified enzyme solution was placed at room temperature under 5%, 10%, 15% and 18% NaCl solutions respectively, and samples were taken at different time points to measure the enzyme activity. The method for measuring enzyme activity is: 100mM Tris-HCl buffer solution (pH7.2), with 1mM p-nitrophenol butyrate as substrate, add 20 μl of sample enzyme solution, and measure the absorbance value A405nm at 35°C. The result is as figure 2 As shown, the recombinant esterase EST_115 can still maintain 40% of the enzyme activity when stored at 18% NaCl concentration for 30 days. The results showed that the recombinant esterase EST_115 had strong salt tolerance.
PUM
| Property | Measurement | Unit |
|---|---|---|
| absorbance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com