Folate-modified chondroitin sulfate-deoxycholic acid polymer and its synthesis method and application
A technology of chondroitin sulfate and deoxycholic acid, which is applied in the direction of non-active ingredient medical preparations, medical preparations containing active ingredients, drug combinations, etc., to achieve low toxicity and side effects, excellent biocompatibility, and round shape Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
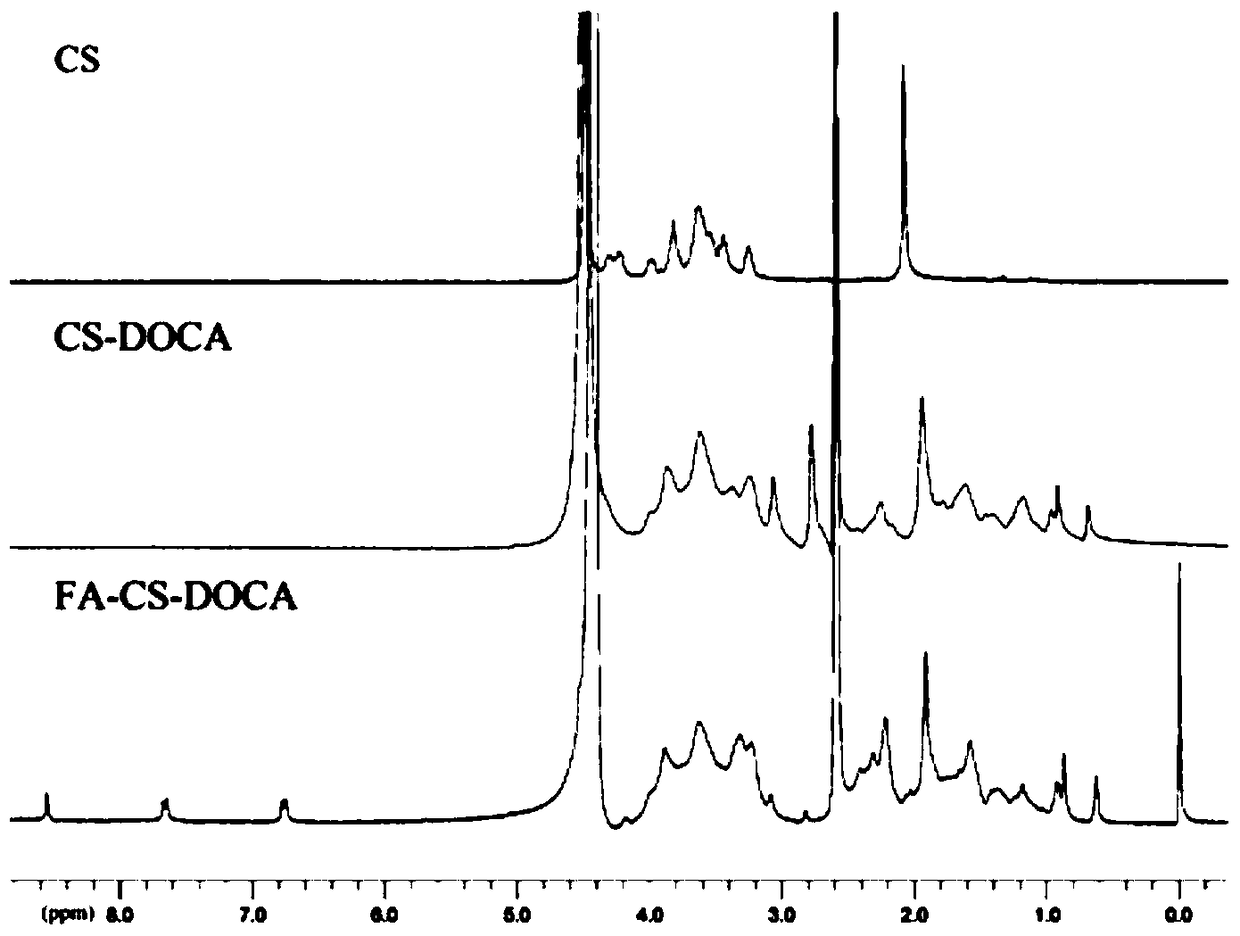
[0052] Embodiment 1: the synthesis of chondroitin sulfate-deoxycholic acid polymer
[0053] (1) Synthesis of chondroitin sulfate-adipate dihydrazide: Weigh 0.5g chondroitin sulfate and dissolve it in 150mL distilled water, stir to make it fully swell and dissolve, then add 5.0g adipic acid dihydrazide to the solution in turn Hydrazide, 1.6g 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride and 0.23g N-hydroxysuccinimide, adjust the pH value of the reaction system to 6.8 with sodium hydroxide , reacted at room temperature for 24 hours, dialyzed with distilled water for three days, and freeze-dried to obtain the intermediate product chondroitin sulfate-adipate dihydrazide.
[0054] (2) Weigh 80 mg of deoxycholic acid and dissolve it in 5 mL of N,N-dimethylformamide, and sequentially add 1-(3-dimethylaminopropyl)-3- Ethylcarbodiimide hydrochloride, N-hydroxysuccinimide equivalent to 2 times the molar amount of deoxycholic acid, stirred at room temperature for 30 minute...
Embodiment 2
[0057] Embodiment 2: Synthesis of folic acid-modified chondroitin sulfate-deoxycholic acid polymer
[0058] (1) Synthesis of chondroitin sulfate-adipate dihydrazide: Weigh 0.5g chondroitin sulfate and dissolve it in 150mL distilled water, stir to make it fully swell and dissolve, then add 5.0g adipic acid dihydrazide to the solution in turn Hydrazide, 1.6g 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride and 0.23g N-hydroxysuccinimide, adjust the pH value of the reaction system to 6.8 with sodium hydroxide , reacted at room temperature for 24 hours, dialyzed with distilled water for three days, and freeze-dried to obtain the intermediate product chondroitin sulfate-adipate dihydrazide.
[0059] (2) Weigh 80 mg of deoxycholic acid and dissolve it in 5 mL of N,N-dimethylformamide, and sequentially add 1-(3-dimethylaminopropyl)-3- Ethylcarbodiimide hydrochloride, N-hydroxysuccinimide equivalent to 2 times the molar amount of deoxycholic acid, stirred at room temperatu...
Embodiment 3
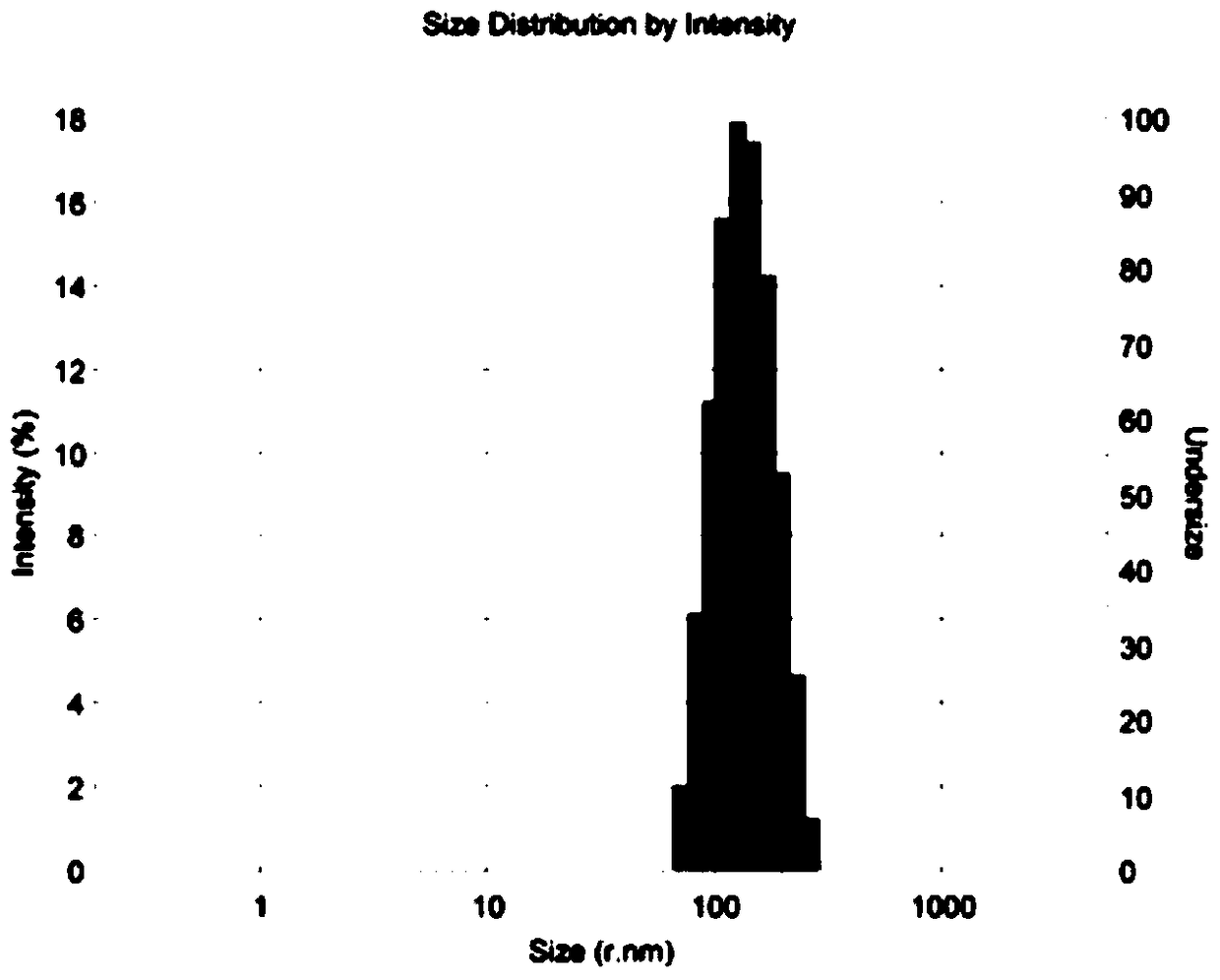
[0064] Example 3: Preparation of folic acid-modified chondroitin sulfate-deoxycholic acid polymer nanomicelles loaded with docetaxel
[0065] 50 mg of folic acid-modified chondroitin sulfate-deoxycholic acid (prepared in Example 2) was ultrasonically dispersed in 10 mL of deionized water for later use; another 15 mg of docetaxel was dissolved in 2 mL of methanol, and slowly added dropwise under strong stirring Add the above-mentioned folic acid-modified chondroitin sulfate-deoxycholic acid polymer aqueous solution, continue to stir vigorously at room temperature overnight (8-12), then use probe-type ultrasonic treatment at 120W power three times, each time for 2min, pulse on for 2s and stop. For 4s, the temperature was kept at 4°C to 8°C, then the solution was transferred to a dialysis bag and dialyzed against water for 24 hours, and then the resulting solution was centrifuged at 4000r / min for 20min to remove unencapsulated drugs. The supernatant is then passed through a 0.8 μ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| degree of substitution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com